 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevention of Hepatitis A Through Active or Passive Immunization: Recommendations of the Advisory Committee on Immunization PracticesSummary This report provides recommendations for use of the newly licensed hepatitis A vaccines (HAVRIX, manufactured by SmithKline Beecham Biologicals, and VAQTA, manufactured by Merck & Company, Inc.) in persons greater than or equal to 2 years of age and updates previous recommendations for use of immune globulin (IG) for protection against hepatitis A (superseding MMWR 1990;39{No. RR-2}:1-5). For preexposure protection, hepatitis A vaccine can now be used instead of IG in many circumstances; for postexposure prophylaxis, the recommendations for IG use are unchanged. INTRODUCTION Until recently, the primary methods used for preventing hepatitis A have been hygienic measures and passive immunization with immune globulin (IG) to provide short-term preexposure or postexposure protection (1). The ability to grow hepatitis A virus (HAV) in cell culture has resulted in the development of vaccines that prevent HAV infection following preexposure immunization (2-4). For the individual, active immunization can provide long-term protection against HAV infection; from a public health perspective, active immunization provides the means to effectively control this disease. The similarities between the epidemiology of hepatitis A and poliomyelitis suggest that widespread vaccination of appropriate susceptible populations can substantially lower disease incidence, eliminate virus transmission, and, ultimately, eradicate HAV infection. FEATURES OF HEPATITIS A Clinical Illness HAV, a 27-nm RNA agent classified as a picornavirus, can produce either asymptomatic infection or symptomatic infection in humans after an average incubation period of 28 days (range: 15-50 days) (5). The illness caused by HAV infection typically has an abrupt onset that can include fever, malaise, anorexia, nausea, abdominal discomfort, dark urine, and jaundice. The likelihood of having symptoms with HAV infection is related to the person's age. In children less than 6 years of age, most (70%) infections are asymptomatic; if illness does occur, it is not usually accompanied by jaundice (6). Among older children and adults, infection is usually symptomatic, with jaundice occurring in greater than 70% of patients (7). Signs and symptoms usually last less than 2 months, although 10%-15% of persons have prolonged or relapsing disease lasting up to 6 months (8). In persons who have either symptomatic or asymptomatic infection, HAV replicates in the liver, is excreted in bile, and is shed in the stool. Peak infectivity of infected persons occurs during the 2-week period before onset of jaundice or elevation of liver enzymes, when the concentration of virus in stool is highest (9,10). The concentration of virus in stool declines after jaundice appears (9,10). Children and infants can shed HAV for longer periods than do adults, up to several months after the onset of clinical illness (11). Chronic shedding of HAV in feces does not occur; however, shedding may occur in persons who have relapsing illness (12). Diagnosis Hepatitis A cannot be differentiated from other types of viral hepatitis based on clinical or epidemiologic features alone. Serologic testing to detect IgM antibody to the capsid proteins of HAV (IgM anti-HAV) is required to confirm a diagnosis of acute HAV infection. In most persons, IgM anti-HAV becomes detectable 5-10 days after exposure and can persist for up to 6 months after infection (13). IgG anti-HAV, which appears early in the course of infection, remains detectable for the person's lifetime and confers lifelong protection against the disease (14). Commercial diagnostic tests are available for the detection of IgM and total (IgM and IgG) anti-HAV in serum. EPIDEMIOLOGY OF HAV INFECTION Routes of Transmission HAV infection is acquired primarily by the fecal-oral route by either person-to-person contact or ingestion of contaminated food or water. Viremia occurs during the prodromal phase of the illness, and HAV has been transmitted on rare occasions by transfusion (15). In experimentally infected animals, HAV has been detected in saliva during the incubation period; however, transmission by saliva has not been demonstrated (16). Depending on conditions, HAV can be stable in the environment for months (17). Heating foods at temperatures greater than 185 F (85 C) for 1 minute or disinfecting surfaces with a 1:100 dilution of sodium hypochlorite (i.e., household bleach) in tap water is necessary to inactivate HAV (18). Surveillance and Seroprevalence Data In the United States, cyclic increases in the incidence of hepatitis A have occurred approximately every decade; the last nationwide increase occurred in 1989 (19). Between epidemics, hepatitis A continues to occur at relatively high rates. In 1994, a total of 26,796 cases were reported to CDC's National Notifiable Diseases Surveillance System (NNDSS) (20). After the data were corrected for underreporting and asymptomatic infections, an estimated 80,000 cases and 134,000 infections occurred in 1994 (CDC, unpublished data). Hepatitis A incidence varies by race/ethnicity, with highest rates among American Indians/Alaskan Natives and lowest rates among Asians; rates among Hispanics are higher than among non-Hispanics (Figure_1). Rates also are substantially higher in the western United States than in other U.S. regions. Racial/ethnic and geographic differences in rates most likely reflect differences in socioeconomic levels and resultant living conditions (e.g., crowding), which facilitate transmission of HAV. The highest rates of hepatitis A are among children 5-14 years of age (Figure_2). Almost 30% of reported hepatitis A cases occur among children less than 15 years of age (21). Presumably many more children have unrecognized, asymptomatic infection and can be a source of infection for others (6,11). The most frequently reported source of infection (22%-26%) is either household contact or sexual contact with a person who has hepatitis A (21). An additional 14%-16% of reported cases occur among children or employees in day care centers or among contacts of children or employees in day care centers; 4%-6% occur among international travelers; and another 2%-3% are associated with recognized food or waterborne disease outbreaks (19,21). Person-to-person contact is thought to be the source of infection in outbreaks among injecting- and noninjecting-drug users and among men who have sex with men (22,23). Approximately 50% of persons with hepatitis A do not have an identified source of infection (21). In the United States, 33% of the population has serologic evidence of prior HAV infection as determined by Phase 1 of the Third National Health and Nutrition Examination Survey (NHANES-III) conducted during 1988-1991 (CDC, unpublished data). Anti-HAV prevalence is directly related to age: among children less than 10 years of age, the prevalence is 10%; 20-29 years of age, 18%; 40-49 years of age, 49%; and greater than 70 years of age, 75%. Anti-HAV prevalence is highest among Mexican-Americans (67%), compared with blacks (37%) and whites (29%). Anti-HAV prevalence is inversely related to income. Hepatitis A results in substantial morbidity with associated costs caused by medical care and work loss; 11%-22% of persons who have hepatitis A are hospitalized (21). Adults who become ill lose an average of 27 days of work per illness (Table_1). Health departments incur substantial costs in providing postexposure prophylaxis to an average of 11 contacts per case (CDC, unpublished data). Average costs (direct and indirect) of hepatitis A range from $1,817 to $2,459 per case for adults and from $433 to $1,492 per case for children less than 18 years of age (Table_1). In 1989, the estimated annual cost (direct and indirect) of hepatitis A in the United States was greater than $200 million (24). In the United States, an estimated 100 deaths occur each year as a result of fulminant hepatitis A. Data reported to CDC indicate that the case-fatality rate among persons of all ages is approximately 0.3%. However, the risk for death is 1.8% among adults greater than 50 years of age; persons who have chronic liver disease have a high risk of death from fulminant hepatitis A (25,26). Communitywide Outbreaks In the United States, most hepatitis A occurs through person-to-person transmission during communitywide outbreaks (27) when the highest rates of disease occur among children, adolescents, and young adults. Outbreaks have been difficult to control despite enhanced awareness of preventive measures and postexposure prophylaxis with IG. Experience during most communitywide outbreaks has indicated that widespread postexposure prophylaxis with IG may slow HAV transmission but does not stop the outbreak (28,29). Categorizing communities that experience hepatitis A outbreaks can help determine whether to use hepatitis A vaccine to control or prevent communitywide outbreaks. Communities that experience hepatitis A outbreaks can be considered as either communities that have high rates of hepatitis A or communities that have intermediate rates of hepatitis A, based on certain epidemiologic characteristics (e.g., age-specific rates of infection and temporal patterns of disease incidence) (Table_2). In both types of communities, outbreaks usually have been difficult to control. Communities That Have High Rates of Hepatitis A Communities that have high rates of infection typically have epidemics of hepatitis A every 5-10 years that may last for several years, have high rates of disease, and have few cases among persons greater than 15 years of age (Table_2). Seroprevalence data indicate that 30%-40% of children in these communities acquire infection before 5 years of age and almost all persons become infected before reaching young adulthood (29-31). These communities often are relatively well defined, either geographically or ethnically, and include American Indian, Alaskan Native, Pacific Islander, and selected Hispanic and religious communities (29,30,32-34). Communities That Have Intermediate Rates of Hepatitis A In communities that have intermediate rates of hepatitis A, most disease occurs among children, adolescents, and young adults, in contrast to communities that have high rates of hepatitis A, in which the majority of cases occur among children less than 15 years of age. Communities that have intermediate rates of hepatitis A often are large metropolitan areas, and cases may be concentrated in specific census tracts or neighborhoods (Table_2) (28,35). Overall disease rates during epidemic periods typically range from 50 to 200 cases/100,000 population per year; however, within some census tracts, disease rates can be as high as those in communities that have high rates of hepatitis A. Surveillance data indicate epidemics often occur at regular intervals and persist for several years. However, some communities that have intermediate rates of hepatitis A do not have periodic epidemics but instead have sustained elevated rates of disease for many years. The epidemiologic factors associated with these differences in disease patterns have not been determined. During epidemic periods, hepatitis A rates generally increase among all age groups, indicating widespread disease within the community (36). Occasionally during outbreaks, the number of cases may increase among users of illegal drugs, men who have sex with men, or children and employees in day care centers (6,36-38). By examining local surveillance data, each community can determine if such groups represent a substantial source of HAV infection. Data from some studies indicate that children with asymptomatic HAV infection can be a substantial source of infection for older persons during communitywide outbreaks. Data from a study in California among adults without an identified source of infection indicate that 25% of their asymptomatic contacts less than 6 years of age were IgM anti-HAV positive (CDC, unpublished data). Groups at Increased Risk for Hepatitis A The following groups are at increased risk for hepatitis A: Travelers Persons from developed countries who travel to developing countries are at substantial risk for acquiring hepatitis A (39). Such persons include tourists, military personnel, missionaries, and others who work or study abroad in countries that have high or intermediate endemicity of hepatitis A (Figure_3). Data from prospective studies indicate that the risk among travelers who do not receive IG is 3/1,000-5/1,000 per month of stay; among some travelers, the risk is higher (40). The risk varies according to region visited and the length of stay. The risk for hepatitis A is increased even among travelers who report that they observe measures to protect themselves against enteric infection or stay only in urban areas or luxury hotels, or both (CDC, unpublished data). Men Who Have Sex with Men Hepatitis A outbreaks among men who have sex with men have been reported frequently. Recent outbreaks have occurred in urban areas in the United States, Canada, and Australia (23). Data from prospective serosurveys have demonstrated rates of HAV infection among men who have sex with men that are several-fold higher than those among control populations (41,42). Injecting-Drug Users During the past decade, outbreaks have been reported among injecting-drug users in the United States and in Europe (22,37,38). In the late 1980s, 10%-19% of persons who had hepatitis A reported a history of injecting-drug use; however, in recent years, less than 3% of infected persons have reported this behavior (21). Persons Working with Nonhuman Primates Outbreaks of hepatitis A have been reported among persons working with non- human primates that are susceptible to HAV infection, including several Old World and New World species (43,44). Primates that were infected were those that had been born in the wild, not those that had been born and raised in captivity. Risk for Hepatitis A Among Other Groups and Settings Persons Who Have Chronic Liver Disease Although not at increased risk for HAV infection, persons who have chronic liver disease are at increased risk for fulminant hepatitis A (45). Data from death certificates indicate a high prevalence of chronic liver disease among persons who had fulminant hepatitis A (46). Persons Who Have Clotting-Factor Disorders During 1992-1993, several outbreaks of hepatitis A were reported in Europe among persons who had clotting-factor disorders who had been administered solvent- detergent-treated factor VIII concentrates that presumably had been contaminated from plasma donors incubating hepatitis A (47). In the United States, data from one serologic study suggested that hemophilic patients may be at increased risk for HAV infection (48). During 1995-1996, several patients who had clotting-factor disorders reportedly developed hepatitis A after having been administered solvent-detergent-treated factor VIII and factor IX concentrates (49). Food-Service Establishments/Food Handlers Foodborne hepatitis A outbreaks are relatively uncommon in the United States; however, when they occur, intensive public health efforts are required for their control. These outbreaks are usually associated with contamination of food during preparation by an HAV-infected food handler (50), although food (e.g., shellfish) that has been contaminated before reaching the food-service establishment has been associated with some outbreaks (51-53). Although persons who work as food handlers have a critical role in common-source foodborne HAV transmission, they are not at increased risk for hepatitis A because of their occupation. In a study of hepatitis A cases in Washington State during 1987-1988, rates of hepatitis A among food handlers were found to be similar to rates among the general population in the state (Trueman Sharp, University of Washington, unpublished data). Day Care Centers Outbreaks among children attending day care centers and persons employed at these centers have been recognized since the 1970s (54). Because infection among children is usually mild or asymptomatic, outbreaks often are recognized only when adult contacts (usually parents) become ill (6). Poor hygiene among children who wear diapers and the handling and changing of diapers by staff contribute to the spread of HAV infection; outbreaks rarely occur in day care centers in which care is provided only to children who do not wear diapers. Despite the occurrence of outbreaks when HAV is introduced into day care centers, the results of serologic surveys do not indicate a substantially increased prevalence of HAV infection among staff at day care centers compared with the prevalence among control populations (55,56). Furthermore, the NHANES-III study did not indicate an increased prevalence of HAV infection among children and adolescents who previously attended day care centers (CDC, unpublished data). Although outbreaks at day care centers occasionally are the sources of outbreaks of hepatitis A within a community, disease within day care centers more commonly reflects extended transmission in the community. Health-Care Institutions Nosocomial HAV transmission is rare. Outbreaks have occasionally been observed in neonatal intensive-care units because of infants acquiring infection from transfused blood and subsequently transmitting hepatitis A to other infants and staff (11,57,58). Outbreaks of hepatitis A caused by transmission from adult patients to health-care workers are usually associated with fecal incontinence, although most hospitalized patients who have hepatitis A are admitted after onset of jaundice when they are beyond the point of peak infectivity (59). Data from serologic surveys of many types of health-care workers have not indicated an increased prevalence of HAV infection in these groups compared with that in control populations (60-62). Institutions for Persons Who Have Developmental Disabilities Historically, HAV infection was highly endemic in institutions for persons who have developmental disabilities (63). As fewer children have been institutionalized and conditions within institutions have improved, the incidence and prevalence of HAV infection have decreased, although sporadic outbreaks can occur in these settings (51). Schools In the United States, the occurrence of cases of hepatitis A within elementary or secondary schools usually reflects disease acquisition within the community. Child-to-child disease transmission within the school setting is uncommon; thus, if multiple cases occur among children at a school, the possibility of a common source of infection should be investigated (51). Workers Exposed to Sewage Data from serologic studies among Scandinavian and English workers who had been exposed to sewage indicate a possible elevated risk for HAV infection; however, in these studies, the data were not controlled for other risk factors (e.g., socioeconomic status) (64,65). In the United States, no work-related cases of HAV transmission have been reported among workers exposed to sewage, and serologic data are not available. Other Settings Waterborne outbreaks of hepatitis A are infrequent. Most outbreaks are associated with sewage-contaminated water or inadequately treated water (66,67). Surveillance for Hepatitis A Hepatitis A is a reportable disease in all states. The goals of hepatitis A surveillance at the national, state, and local levels include a) monitoring disease incidence by identifying acute, symptomatic infections in all age groups; b) determining the epidemiologic characteristics of infected persons, including the source of infection; c) identifying contacts of case-patients who might require postexposure prophylaxis; d) detecting outbreaks; e) determining the effectiveness of hepatitis A vaccination; and f) determining missed opportunities for vaccination. Cases of hepatitis A should be reported to local or state health departments (according to specific state requirements) so that appropriate control measures can be implemented, if indicated. Cases meeting specified criteria are reported by state health departments to CDC (68). Hepatitis A surveillance must be maintained at the local level so that the various immunization strategies recommended in this report can be implemented and their outcome at the local, state, and national levels can be assessed. RATIONALE FOR PREVENTION OF HEPATITIS A THROUGH ACTIVE IMMUNIZATION In the United States during the past several decades, a decline in the overall incidence of hepatitis A has occurred primarily as a result of better hygienic and sanitary conditions (e.g., improved water supplies, sewage disposal, and food sanitation and less crowded living conditions). Although passive immunization with IG has been available for several decades, its effect on lowering the incidence of hepatitis A has been limited. High rates of disease among many segments of the U.S. population and the continued occurrence of extensive communitywide outbreaks indicate that hepatitis A remains a major public health problem. The availability of hepatitis A vaccine provides an opportunity to substantially lower disease incidence and eventually eradicate infection. This reduction in disease incidence will be achieved by producing high levels of immunity in persons in age groups that have the highest rates of HAV infection and that serve as a reservoir of infection (26). Producing a highly immune population decreases the incidence of hepatitis A and presumably decreases virus circulation by preventing fecal shedding of HAV. Populations are highly immune following an epidemic in communities that have high rates of hepatitis A or following vaccination in such communities (29). Hepatitis A immunization is likely to substantially lower disease incidence because HAV does not produce a chronic infection, and humans are the only natural reservoir of the virus. Because of their critical role in HAV transmission, children should be a primary focus of immunization strategies to lower disease incidence. Thus, the most effective means of achieving control of HAV infection would be to include routine hepatitis A vaccination in the childhood vaccination schedule. However, the lack of data available for determining the appropriate dose and timing of vaccination in the first or second year of life presents a barrier to the implementation of this strategy. Combination vaccines that include inactivated HAV would minimize the number of injections administered to children. Until hepatitis A vaccine is licensed for use among children less than 2 years of age, the interim strategy to prevent and control hepatitis A should focus on preexposure vaccination of the following persons: a) persons at increased risk for HAV infection or its consequences (e.g., travelers and persons who have chronic liver disease); b) children living in communities that have high rates of hepatitis A to help prevent recurrent epidemics; and, if indicated, c) children and young adults in communities that have intermediate rates of hepatitis A to help control ongoing and prevent future epidemics. In addition, contacts of case-patients should be administered postexposure prophylaxis (i.e., IG or, when appropriate, IG and hepatitis A vaccine). Vaccination of persons in groups at increased risk for HAV infection (e.g., travelers) will likely have little effect on national disease rates because most cases do not occur among persons in these groups. Vaccination of persons in communities with high and intermediate rates of disease might have an impact on national disease incidence. However, a substantial reduction in the incidence of disease cannot be expected until hepatitis A vaccine is included in the routine childhood immunization schedule and successive cohorts of children are vaccinated. PROPHYLAXIS AGAINST HEPATITIS A VIRUS INFECTION Immune Globulin IG is a sterile preparation of concentrated antibodies (immunoglobulins) made from pooled human plasma processed by cold ethanol fractionation (69). In the United States, only plasma that has tested negative for a) hepatitis B surface antigen (HBsAg), b) antibody to human immunodeficiency virus (HIV), and c) antibody to hepatitis C virus (HCV) is used to manufacture IG. Cold ethanol fractionation can eliminate and inactivate HIV (70). Furthermore, no transmission of hepatitis B virus, HIV, HCV, or other viruses has been reported from the intramuscular (IM) administration of IG (71). Anti-HAV titers differ between IG lots, and slightly lower titers have been observed in recent years, probably because of the decreasing prevalence of HAV infection among plasma donors (72). However, no clinical or epidemiologic evidence of decreased protection has been observed. IG provides protection against hepatitis A through passive transfer of antibody. The levels of anti-HAV achieved following IM administration of IG are below the level of detection of most commercially available diagnostic tests (73). When administered for preexposure prophylaxis, a dose of 0.02 mL/kg IM confers protection for less than 3 months, and a dose of 0.06 mL/kg IM confers protection for less than or equal to 5 months (Table_3). When administered within 2 weeks following an exposure to HAV, IG is greater than 85% effective in preventing hepatitis A (74-76). Efficacy is greatest when IG is administered early in the HAV incubation period; when administered later in the incubation period, IG often only attenuates the clinical expression of HAV infection (74). For administration of IG, an appropriate muscle mass (i.e., the deltoid or gluteal muscle) should be chosen into which a large volume of IG can be injected by using a needle length appropriate for the person's age and size (77). If a gluteal muscle is used, the central region of the buttock should be avoided: only the upper outer quadrant should be used, and the needle should be directed anteriorly to minimize the possibility of injury to the sciatic nerve (77). Serious adverse events from IG are rare. Anaphylaxis has been reported after repeated administration to persons who have known IgA deficiency; thus, IG should not be administered to these persons (78). Pregnancy or lactation is not a contraindication to IG administration. IG does not interfere with the immune response to oral poliovirus vaccine or yellow fever vaccine, or, in general, to inactivated vaccines. However, IG can interfere with the response to live, attenuated vaccines (e.g., measles, mumps, rubella, and varicella) when vaccines are administered either individually or as combination vaccines. Administration of these vaccines should be delayed for at least 5 months after administration of IG for hepatitis A prophylaxis. IG should not be administered within 2 weeks after the administration of live, attenuated vaccines (or within 3 weeks after varicella vaccine) unless the benefits of IG administration exceed the benefits of vaccination (77). If IG is administered within 2 weeks after administration of these vaccines (or within 3 weeks after administration of varicella vaccine), the person should be revaccinated, but not sooner than 5 months after the administration of IG (77). Hepatitis A Vaccine Several inactivated and attenuated hepatitis A vaccines have been developed and evaluated in human clinical trials and in primate models of HAV infection (79); however, only inactivated vaccines have been evaluated for efficacy in controlled clinical trials (3,4). The vaccines currently licensed in United States are HAVRIX (manufactured by SmithKline Beecham Biologicals) and VAQTA (manufactured by Merck & Company, Inc). Both are inactivated vaccines. Preparation Inactivated hepatitis A vaccine is prepared by methods similar to those used for inactivated poliovirus vaccine (80,81). Cell-culture-adapted virus is propagated in human fibroblasts, purified from cell lysates by ultrafiltration and exclusion gel chromatography or other methods, formalin inactivated, adsorbed to an aluminum hydroxide adjuvant, and prepared with 2-phenoxyethanol (for HAVRIX ) as a preservative; VAQTA is formulated without a preservative. For HAVRIX, the antigen content of the final aqueous preparation is determined by reactivity in a quantitative immunoassay for HAV antigen, and final vaccine potency (per dose) is expressed as enzyme-linked immunosorbent assay (ELISA) units (EL.U.). For VAQTA, the antigen content is expressed as units (U) of hepatitis A antigen. Vaccine Storage and Shipment Hepatitis A vaccine should be stored and shipped at temperatures ranging from 35.6 F (2 C) to 46.4 F (8 C) and should not be frozen. However, the reactogenicity and immunogenicity of HAVRIX and VAQTA after storage at 98.6 F (37 C) for 1 week do not differ from those of vaccines stored at the recommended temperature (82; Merck & Company, Inc., unpublished data). Route of Administration, Vaccination Schedule, and Dosage The vaccine should be administered intramuscularly into the deltoid muscle. A needle length appropriate for the vaccinee's age and size should be used (77). HAVRIX is currently licensed in three formulations, and the formulation and number of doses differ according to the vaccinee's age: for persons 2-18 years of age, 360 EL.U. per dose in a three-dose schedule and 720 EL.U. per dose in a two-dose schedule; for persons greater than 18 years of age, 1,440 EL.U. per dose in a two-dose schedule (Table_4). VAQTA is licensed in two formulations, and the formulation and number of doses differ according to the person's age: for persons 2-17 years of age, 25 U in a two-dose schedule; for persons greater than 17 years of age, 50 U per dose in a two-dose schedule (Table_5). Vaccine Performance Detection of anti-HAV after vaccination. Concentrations of antibody achieved after passive transfer by IG or active induction by vaccination are 10-100-fold lower than those produced after natural infection and are often below the detection level of standard, commercially available assays (73). To measure lower levels of antibody, more sensitive immunoassays have been developed that correlate more closely with neutralizing antibody assays (73). The anti-HAV immunoassays commercially available in the United States can be modified to detect lower concentrations of antibody; however, the modified assays have not been reviewed by the U.S. Food and Drug Administration and are not approved for any clinical indication. Anti-HAV concentrations are measured in comparison with a World Health Organization reference immunoglobulin reagent and are expressed as milli-International Units per milliliter (mIU/mL). The lower limits of detection are approximately 100 mIU/mL by unmodified, commercially available assays and 10-12 mIU/mL by modified assays. Thus, a positive anti-HAV result by a standard assay indicates protection. However, after vaccination, persons who are anti-HAV negative by standard assays might still have protective levels of antibody. The absolute lower limit of antibody required to prevent HAV infection has not been defined. In vitro studies using cell-culture-derived virus indicate that low levels of antibody (e.g., less than 20 mIU/mL) can be neutralizing (83). Clinical studies have yielded few data from which a minimum protective antibody level can be derived because vaccine-induced levels of antibody have been high and few infections have been detected among vaccinated persons. Experimental studies in chimpanzees indicate that low levels of passively transferred antibody (less than 10 mIU/mL) obtained from immunized persons do not protect against infection but do prevent clinical hepatitis and virus shedding (84). To define a protective antibody response, most clinical studies conducted with HAVRIX have been based on levels greater than 20 mIU/mL as measured with a modified enzyme immunoassay, and studies conducted with VAQTA have been based on levels greater than 10 mIU/mL as measured with a modified radioimmunoassay (85,86). Immunogenicity in adults. HAVRIX is highly immunogenic in persons greater than or equal to 18 years of age if two doses of 1,440 EL.U. are administered on a 0- and 6-to-12-month schedule (86). Anti-HAV levels greater than 20 mIU/mL developed in 88% (range: 80%-98%) of adults 15 days after the first dose and in 99%-100% of adults at 1 month. Among a sample of vaccinees, 54%-62% of persons were positive for neutralizing antibody 14 days after the first dose, and 94%-100% of persons were positive at 1 month (86; SmithKline Beecham Biologicals, unpublished data). After the second dose, all persons had protective levels of antibody (greater than 20 mIU/mL) with a high geometric mean titer (GMT) (Table_6), and all were positive for neutralizing antibody (SmithKline Beecham Biologicals, unpublished data). VAQTA provides similar immunogenicity when administered to adults greater than or equal to 18 years of age (Table_7). Among vaccinated persons who received 50 U at 0 and 6 months, 95% had protective anti-HAV levels at 1 month, and 100% had protective levels at 7 months. The GMT, measured by using a modified hepatitis A antibody (HAVAB) assay, was 37 mIU/mL at 1 month and 5,059 mIU/mL at 7 months (87). Immunogenicity in children and adolescents. Of persons 1-17 years of age who were administered three doses of 360 EL.U. of HAVRIX on a 0-, 1-, and 6-month schedule, 95% developed protective levels of anti-HAV 1 month after the first dose (86,88-90). One month after the second dose, all persons had protective levels of antibody that persisted until administration of the third dose at 6 months. One month following the third dose, the GMT increased approximately tenfold and persisted at high levels 6 months later (Table_6). Among children and adolescents 2-18 years of age who were administered two doses of HAVRIX (720 EL.U. per dose at 0- and 6-month intervals), 99% had protective levels of antibody 1 month after receiving the first dose (Table_6). Similar 1-month results were obtained for adolescents who had been administered two doses of HAVRIX (1,440 EL.U. per dose). When administered to persons 2-17 years of age in a variety of two-dose schedules (25 U per dose), VAQTA was highly immunogenic. From 97% to 100% of children had protective levels 1 month after the first dose, and 100% had protective levels 1 month after administration of a second dose at 6, 12, or 18 months, with substantial increases in GMT after administration of the second dose (Table_7) (87). Immunogenicity in infants. Few data are available regarding the use of hepatitis A vaccine in children less than 2 years of age. Results from one study indicated that among infants without passively acquired maternal anti-HAV who had been administered hepatitis A vaccine (360 EL.U. per dose) at 2, 4, and 6 months of age, 100% of the infants had protective antibody levels with a GMT of 794 mIU/mL 1 month following the third dose (91) (Table_6). Infants with passively transferred maternal anti-HAV had a reduced anti-HAV GMT after vaccination (see Factors Associated with Reduced Immunogenicity). IgM anti-HAV after vaccination. Hepatitis A vaccination rarely induces IgM anti-HAV that is detectable by standard assays. In one study, three of approximately 311 adult vaccine recipients transiently developed IgM anti-HAV 1 month after completing vaccination with 720 EL.U. of HAVRIX on a 0-, 1-, and 6-month schedule (92). In another study, none of 158 children studied had detectable IgM anti-HAV 1 month after receiving two doses of HAVRIX (360 EL.U. per dose) (SmithKline Beecham Biologicals, unpublished data). IgM anti-HAV was detected in three of 15 persons at 2-3 weeks after having been administered VAQTA (93). Efficacy. The efficacy of HAVRIX was evaluated in a double-blind, placebo- controlled, randomized clinical trial conducted in Thailand among approximately 40,000 children 1-16 years of age living in villages that had high rates of hepatitis A (4). After two doses of vaccine (360 EL.U. per dose) administered 1 month apart, the efficacy of vaccine in protecting against clinical hepatitis A was 94% (95% confidence interval=79%-99%). A double-blind, placebo-controlled, randomized clinical trial using VAQTA was conducted among approximately 1,000 children 2-16 years of age living in a New York community that had a high rate of hepatitis A. The protective efficacy against clinical hepatitis A was 100% after administration of one dose (25 U) of vaccine (3). Studies of chimpanzees indicate that hepatitis A vaccine can prevent HAV infection if administered shortly after exposure (94). Because the incubation period of hepatitis A can be less than or equal to 50 days, the fact that no cases of hepatitis A occurred in vaccine recipients beginning 19 days after vaccination indicates a possible postexposure effect (3). A study comparing the postexposure efficacy of hepatitis A vaccine versus IG has not been done. Effectiveness in outbreak settings. Several studies have examined the effectiveness of hepatitis A vaccine in controlling outbreaks in communities that have high rates of hepatitis A. Specifically, vaccination using VAQTA of children 2-16 years of age during the clinical trial evaluating vaccine efficacy resulted in a substantial decrease in community hepatitis A rates (3). In addition, in several Alaskan villages in which hepatitis A outbreaks were occurring, vaccination of susceptible persons less than 30 years of age with one dose of HAVRIX (720 EL.U.) resulted in a rapid decrease in the number of cases (95). Both studies were carried out in small, well-defined communities in which an estimated 70% or more of the susceptible persons were vaccinated. Cost analyses have indicated that vaccination in communities that have high rates of hepatitis A can be cost-saving (96). Hepatitis A vaccine has been used in several communities that had intermediate rates of hepatitis A and were experiencing outbreaks. In Butte County, California, hepatitis A cases decreased concurrently with the implementation of a program in which approximately 37% of children 2-12 years of age were administered one dose of VAQTA (97). In Memphis, Tennessee, following a targeted vaccination program in which one dose of HAVRIX (360 EL.U.) was administered to 52% of eligible children 2-9 years of age, hepatitis A rates decreased in this target population (98). In two villages in Slovakia, a communitywide outbreak ended 2 months after approximately two thirds of school-age children were vaccinated with two doses of HAVRIX (99). Further study is needed to determine the effectiveness of this strategy, the feasibility of implementation, and level of vaccination coverage required to interrupt disease transmission. Long-term protection. Data concerning the long-term persistence of antibody and of immune memory are limited because the currently available vaccines have been under evaluation for only 4-5 years. Among adults who received three doses of HAVRIX (720 EL.U. per dose at 0-, 1-, and 6-month intervals), 100% of those persons had anti-HAV levels greater than 20 mIU/mL 48 months after the initial dose, although antibody concentrations had decreased by approximately 50% (P. Van Damme, University of Antwerp {Belgium}, unpublished data). Data regarding persons who were administered VAQTA and who were monitored for 36 months also demonstrated a decrease in titer; however, protective levels of anti-HAV were still observed in 100% of these persons (100). Estimates of antibody persistence derived from kinetic models of antibody decline indicate that protective levels of anti-HAV could be present for greater than or equal to 20 years (100,101). Whether other mechanisms (e.g., cellular memory) also contribute to long-term protection is unknown. The long-term protective efficacy of hepatitis A vaccine needs to be determined in ongoing studies to detect clinical illness among vaccinees and in future postmarketing surveillance studies before recommendations can be made concerning the possible need for booster doses. Factors associated with reduced immunogenicity. In one study, the percentage of adults who were administered IG concurrently with the first dose of hepatitis A vaccine and who had protective levels of antibody was similar to the percentage of adults who had protective levels and who had been administered hepatitis A vaccine alone; however, their GMTs were substantially lower 1 month after being administered three doses of HAVRIX (720 EL.U. per dose) than GMTs of adults who had been administered hepatitis A vaccine alone (GMT 2,488 MIU/mL versus 3,614 mIU/mL, respectively) (102). In both groups, the antibody levels were at least 100-fold higher than levels considered to be protective. A similar effect occurred with concurrent administration of IG and VAQTA (Merck & Company, Inc., unpublished data). Therefore, the reduced immunogenicity of hepatitis A vaccine that occurs with concurrent administration of IG is not expected to be clinically significant. Reduced immunogenicity also was observed in infants who had passively acquired antibody because of prior maternal HAV infection (91). Infants who were administered HAVRIX (360 EL.U. per dose) at 2, 4, and 6 months of age and whose mothers were anti-HAV positive had antibody levels at 15 months of age that were one third the levels in infants who had been administered HAVRIX on the same schedule but whose mothers were anti-HAV negative (GMT 84 mIU/mL versus 231 mIU/mL, respectively). However, 93% and 100% of infants in each group, respectively, had anti-HAV levels greater than 20 mIU/mL. In one study, the proportion of persons greater than 40 years of age who had protective antibody levels after three doses of HAVRIX (720 EL.U. per dose) was similar to that of persons less than or equal to 40 years of age, although the final GMTs were approximately 50% lower (SmithKline Beecham Biologicals, unpublished data). Data from one study of HIV- infected persons vaccinated with three doses of 720 EL.U. of HAVRIX indicate that both the proportion of those who developed protective antibody levels and their GMTs were lower than those in anti-HIV negative persons (77% versus 100%; 636 mIU/mL versus 1,687 mIU/mL, respectively) (103). Other factors associated with decreased immunogenicity to other vaccines (e.g., smoking) have not been evaluated for hepatitis A vaccine. No data are available pertaining to response rates to revaccination among persons who do not respond to the primary vaccination series. Simultaneous administration with other vaccines. Limited data from studies conducted among adults indicate that simultaneous administration of hepatitis A vaccine with diphtheria, poliovirus (oral and inactivated), tetanus, oral typhoid, cholera, Japanese encephalitis, rabies, or yellow fever vaccine does not decrease the immune response to either vaccine or increase the frequency of reported adverse events (104; SmithKline Beecham Biologicals, unpublished data). Studies indicate that hepatitis B vaccine can be administered simultaneously with either HAVRIX or VAQTA without either affecting immunogenicity or increasing the frequency of adverse events (105). Several studies are being conducted among infants to evaluate the effect of simultaneous administration of hepatitis A, diphtheria-tetanus-pertussis (DTP), and oral poliovirus vaccines on the immunogenicity and reactogenicity of these vaccines. Side Effects and Adverse Events Data concerning adverse events are derived from prelicensure clinical studies worldwide and from reports following vaccine licensure of HAVRIX in Europe and Asia. Approximately 50,000 persons have been administered HAVRIX in clinical studies. No serious adverse events have been attributed definitively to hepatitis A vaccine. Among adults, the most frequently reported side effects occurring within 3 days after the 1,440 EL.U. dose were soreness at the injection site (56%), headache (14%), and malaise (7%); the incidence of side effects generally has been similar to that of hepatitis B vaccine. In clinical studies among children, the most frequently reported side effects were soreness at the injection site (15%), feeding problems (8%), headache (4%), and injection-site induration (4%). No serious adverse events were reported for approximately 40,000 children who were administered the 360 EL.U. dose of hepatitis A vaccine in the protective efficacy study (4). Approximately 9,200 persons have been administered VAQTA in clinical studies. No serious adverse events were reported among participants in the clinical studies. Among adults, the most frequent side effects that occurred within 5 days following vaccination include tenderness (53%), pain (51%), and warmth (17.3%) at the injection site (53%) and headache (16.1%). Among children, the most common side effects reported were pain (19%), tenderness (17%), and warmth (9%) at the injection site. An estimated 1.3 million persons have been vaccinated with HAVRIX since it was licensed in Europe and Asia. Postlicensure reports of serious adverse events, without regard to causality, received by the vaccine manufacturer have included anaphylaxis, Guillain-Barre syndrome, brachial plexus neuropathy, transverse myelitis, multiple sclerosis, encephalopathy, and erythema multiforme (SmithKline Beecham Biologicals, unpublished data). Most of these events have occurred among adults, and approximately one third have occurred among persons receiving other vaccines concurrently. For serious adverse events for which background incidence data are known (e.g., Guillain-Barre syndrome and brachial plexus neuropathy), the rates for vaccine recipients are not higher than would be expected for an unvaccinated population (CDC, unpublished data). In Europe, the ratio of reported adverse events to the number of doses distributed is similar for the manufacturer's hepatitis A and hepatitis B vaccines (SmithKline Beecham Biologicals, unpublished data). Because VAQTA was recently licensed, postmarketing data are limited. An estimated 20,000 persons have been administered VAQTA since it was licensed in the United States and Germany, and no serious adverse events have been reported (Merck & Company, Inc., unpublished data). Any adverse event suspected to be associated with hepatitis A vaccination should be reported to the Vaccine Adverse Events Reporting System (VAERS). VAERS forms can be obtained by calling 1-800-822-7967. Contraindications and Precautions Hepatitis A vaccine should not be administered to persons with a history of hypersensitivity reactions to alum or, in the case of HAVRIX, to the preservative 2-phenoxyethanol. The safety of hepatitis A vaccination during pregnancy has not been determined; however, because hepatitis A vaccine is produced from inactivated HAV, the theoretical risk to the developing fetus is expected to be low. The risk associated with vaccination should be weighed against the risk for hepatitis A in women who may be at high risk for exposure to HAV. Because hepatitis A vaccine is inactivated, no special precautions need to be taken when vaccinating immunocompromised persons. Prevaccination Serologic Testing for Susceptibility HAV infection produces lifelong immunity to hepatitis A and, presumably, to HAV infection. Vaccination of a person who is immune because of prior infection does not increase the risk for adverse events. In populations that have expected high rates of prior HAV infection, prevaccination testing may be considered to reduce costs by not vaccinating persons who have prior immunity. Testing of children is not indicated because of their expected low prevalence of infection. For adults, the decision to test should be based on a) the expected prevalence of immunity; b) the cost of vaccination compared with the cost of serologic testing (including the cost of an additional visit); and c) the likelihood that testing will not interfere with initiating vaccination. For example, if the cost of screening (including laboratory and office visits) is one third the cost of the vaccine series, then screening potential recipients in populations where the prevalence of infection is likely to be greater than 33% should be cost effective (106). Persons for whom prevaccination testing will likely be most cost effective include adults who were either born in or lived for extensive periods in geographic areas that have a high endemicity of HAV infection (Figure_3); older adolescents and adults in certain population groups (i.e., American Indians, Alaskan Natives, and Hispanics); and adults in certain groups that have a high prevalence of infection (e.g., men who have sex with men). In addition, among older adults, the prevalence may be high enough to warrant prevaccination testing. For example, the anti-HAV prevalence among persons greater than 40 years of age, determined by NHANES-III testing, is generally greater than 33% (regardless of race/ethnicity or income level). Thus, if the cost of screening is one third the cost of the vaccination series, prevaccination testing of any person greater than 40 years of age would likely be cost effective. Commercially available tests for total anti-HAV should be used for prevaccination testing. Postvaccination Testing for Serologic Response Postvaccination testing is not indicated because of the high rate of vaccine response among adults and children. Testing methods that have the sensitivity to detect low anti-HAV concentrations after vaccination are not approved for routine diagnostic use in the United States. RECOMMENDATIONS FOR USE OF HEPATITIS A VACCINE AND IMMUNE GLOBULIN Preexposure Protection Against Hepatitis A Virus Infection Hepatitis A vaccination provides preexposure protection from HAV infection in children and adults. Hepatitis A vaccination is recommended for persons who are at increased risk for infection and for any person wishing to obtain immunity. Populations at Increased Risk for HAV Infection or the Adverse Consequences of Infection
Travelers to North America (except Mexico and Central America), western Europe, Japan, Australia, or New Zealand are at no greater risk for infection than in the United States. Data are not available regarding the risk for hepatitis A for persons traveling to developed areas of the Caribbean, although vaccine or IG should be considered if travel to areas that have questionable sanitation is anticipated. Travelers who are administered vaccine should receive the first vaccine dose at least 4 weeks before travel. Persons can be assumed to be protected by 4 weeks after receiving the first vaccine dose, although a second dose 6-12 months later is necessary for long-term protection. Because protection may not be complete until 4 weeks after vaccination, persons traveling to a high-risk area less than 4 weeks after the initial dose also should be administered IG (0.02 mL/kg), but at a different anatomic injection site. Travelers who are allergic to a vaccine component or who elect not to receive vaccine should receive a single dose of IG (0.02 mL/kg), which provides effective protection against hepatitis A for up to 3 months (Table_3). Travelers whose travel period exceeds 2 months should be administered IG at 0.06 mL/kg; administration must be repeated if the travel period exceeds 5 months (Table_3).
Hepatitis A Vaccination in Outbreak Settings
To determine candidate groups for vaccination, local surveillance and epidemiologic data should be used to define populations (e.g., age groups or risk groups) or areas within the community (e.g., census tracts) that have the highest rates of disease. Factors to consider in deciding whether to vaccinate persons in a certain group include a) the feasibility of rapidly vaccinating the target populations of children, adolescents, or young adults; b) program cost; and c) the ability to sustain ongoing vaccination of young children to maintain high levels of immunity and prevent future epidemics. In some communities, day care centers play a role in sustaining communitywide outbreaks. In this situation, consideration should be given to adding hepatitis A vaccine to the immunoprophylaxis regimen for children and staff in the involved center or centers (see Postexposure Prophylaxis with Immune Globulin) and, possibly, vaccinating children in day care centers where cases of hepatitis A have not been detected. Because experience when using hepatitis A vaccine to control hepatitis A in communities that have intermediate rates of hepatitis A is limited, evaluation of the effectiveness of vaccination should be an essential element of programs in these settings.
Postexposure Prophylaxis with Immune Globulin Persons who have been recently exposed to HAV and who have not previously been administered hepatitis A vaccine should be administered a single IM dose of IG (0.02 mL/kg) as soon as possible, but not greater than 2 weeks after exposure. Persons who have been administered one dose of hepatitis A vaccine at least 1 month before exposure to HAV do not need IG. Because hepatitis A cannot be reliably diagnosed on clinical presentation alone, serologic confirmation of HAV infection in index patients by IgM anti-HAV testing is recommended before postexposure treatment of contacts. Screening of contacts for immunity before giving IG is not recommended because screening is more costly than IG and would delay its administration. IG should be administered to previously unvaccinated persons in the following situations. If hepatitis A vaccine is recommended for a person being given IG, it may be administered simultaneously with IG at a separate anatomic injection site.
FUTURE CONSIDERATIONS Both combination vaccines and new information are needed to achieve the goal of substantially reducing the incidence of hepatitis A through routine vaccination of infants or young children. Issues that should be addressed through clinical trials and other studies include the following:
References
+------------------------------------------------------------------
------+
| Erratum: Vol. 45, No. RR-15
|
|
|
| In the MMWR Recommendations and Reports, "Prevention of
|
| Hepatitis A Through Active or Passive Immunization --
Recommendations |
| of the Advisory Committee on Immunization Practices (ACIP)," the
|
| second full paragraph on page 12 (i.e., the last paragraph in
the |
| section titled "Immune Globulin") contained errors regarding the
|
| recommended delay between administration of immune globulin and
|
| measles-mumps-rubella vaccine. The paragraph should read: "IG
does |
| not interfere with the immune response to either oral poliovirus
|
| vaccine or yellow fever vaccine, or, in general, to inactivated
|
| vaccines. However, IG can interfere with the response to other
|
| live, attenuated vaccines (e.g., measles-mumps-rubella {MMR} and
|
| varicella) when vaccines are administered individually or as
|
| combination vaccines. Administration of MMR should be delayed
for |
| at least 3 months, and varicella vaccine should be delayed for
at |
| least 5 months, after administration of IG for hepatitis A
|
| prophylaxis. IG should not be administered within 2 weeks after
the |
| administration of MMR or within 3 weeks after administration of
|
| varicella vaccine unless the benefits of IG administration
exceed |
| the benefits of vaccination (77). If IG is administered within 2
|
| weeks after administration of MMR or within 3 weeks after
|
| administration of varicella vaccine, the person should be
|
| revaccinated, but not sooner than 3 months after the IG
|
| administration for MMR or 5 months for varicella vaccine (77)."
|
| An additional reference for this information is CDC.
|
| Prevention of varicella: recommendations of the Advisory
Committee |
| on Immunization Practices (ACIP). MMWR 1996;45(no. RR-11).
|
|
|
+------------------------------------------------------------------
------+
TABLE 1. Morbidity associated with hepatitis A, by source of data and year -- United States
=====================================================================================================
Source of Data (Year)
-------------------------------------------
Washington State * Sentinel counties +
Outcome (1989-1990) (1991)
-----------------------------------------------------------------------------------------------------
Hospitalization (%)
All ages 11 10 &
Children and adolescents (<18
years of age) 3 7
Adults (>=18 years of age) 13 14
Mean duration of hospitalization 4 (1-10) 2 (1-5)
days (range), all ages
Outpatient visits per case
Average number (range) 4 (0-18) 3 (1-11)
Work loss per case
Average days (range) 27 (0-180) 12 (0-40)
Contacts administered IG @ per
case
Average number 11 10
Average cost per case
Children and adolescents (<18 $ 433 $1,492
years of age)
Adults (>=18 years of age) $2,459 $1,817
-----------------------------------------------------------------------------------------------------
* TR Eng, CDC, unpublished data; based upon interviews of 144 patients; cost data
based upon a subset of 74 patients with complete cost information.
+ Jefferson County, Alabama; Denver County, Colorado; Pierce County, Washington; CDC, un-
published data.
& Hospitalization data are for all hepatitis cases identified (N=287); other data are based on a
subset (N=76) in which no children <18 years of age were hospitalized.
@ Immune globulin.
=====================================================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Features of communities that have high and intermediate rates of hepatitis A
====================================================================================================================================================================
Age of most Reported annual Populations Examples
Community Anti-HAV prevalence patients incidence * Outbreak periodicity (reference)
--------------------------------------------------------------------------------------------------------------------------------------------------------------------
High rate 30%-40% (<5 yrs of 5-14 yrs 700-1,000 5-10 yrs Well defined Alaskan Native
age) 70%-100% (15 geographically or villages (33)
yrs of age) ethnically American Indian
reservations (29)
Selected Hispanic
communities (34)
Selected religious
communities (3)
Intermediate rate 10%-25% (<5 yrs of 5-29 yrs 50-200 May be periodic Less defined than in Zanesville, OH (28)
age) <50% (15 yrs of high-rate communities Oklahoma (38)
age) St. Louis, MO (35)
Selected religous
communities (32)
--------------------------------------------------------------------------------------------------------------------------------------------------------------------
* Typical reported overall incidence per 100,000 population per year during epidemics, all ages.
====================================================================================================================================================================
Return to top. Figure_3 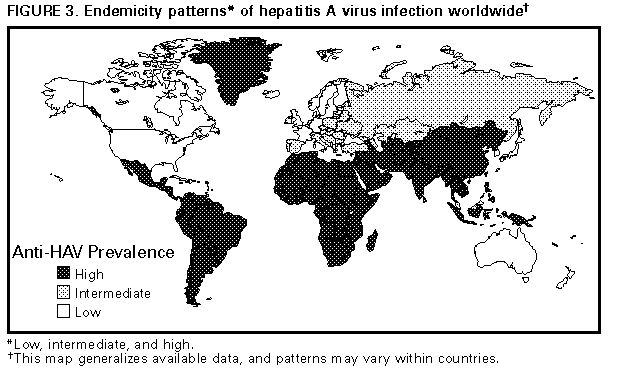 Return to top. Table_3 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 3. Recommended doses of immune globulin (IG) for
hepatitis A preexposure and postexposure prophylaxis
===========================================================
Setting Duration of Coverage IG Dose *
-----------------------------------------------------------
Preexposure Short-term (1-2 mos) 0.02 mL/kg
Long-term (3-5 mos) 0.06 mL/kg +
Postexposure -- 0.02 mL/kg
-----------------------------------------------------------
* IG should be administered by intramuscular injection into
either the deltoid or gluteal muscle. For children <24
months of age, IG can be administered in the
anterolateral thigh muscle.
+ Repeat every 5 months if continued exposure to HAV
occurs.
===========================================================
Return to top. Table_4 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 4. Recommended dosages of HAVRIX *
=======================================================================================
Vaccinee's age (yrs) Dose (EL.U.) + Volume (mL) No. doses Schedule (mos) &
---------------------------------------------------------------------------------------
2-18 720 0.5 2 0, 6-12
18 1,440 1.0 2 0, 6-12
---------------------------------------------------------------------------------------
* Hepatitis A vaccine, inactivated, SmithKline Beecham Biologicals.
+ ELISA units.
& 0 months represents timing of the initial dose; subsequent numbers represent months
after the initial dose.
=======================================================================================
Return to top. Table_5 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 5. Recommended dosages of VAQTA *
=======================================================================
Vaccinee's age Dose (U) + Volume (mL) No. doses Schedule
(yrs) (mos) &
-----------------------------------------------------------------------
2-17 25 0.5 2 0, 6-18
17 50 1.0 2 0, 6
-----------------------------------------------------------------------
* Hepatitis A vaccine, inactivated, Merck & Company, Inc.
+ Units.
& 0 months represents timing of the initial dose; subsequent numbers
represent months after the initial dose.
=======================================================================
Return to top. Table_6 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 6. Summary of selected studies on the immunogenicity of HAVRIX, by age group *
=========================================================================================================================================================
Age Group (yrs) Dose (EL.U.) + Schedule Studies Persons % Seroconversion (GMT)@ by time after receiving first vaccine dose **
(mos) & (No.) (No.) ---------------------------------------------------------------------------
15 days 1 mo 3 mos 6 mos 7 mos 15 mos
---------------------------------------------------------------------------------------------------------------------------------------------------------
Infants ++ 360 2, 4, 6 1 38 100 100
(794) (231)
Children and 360 0, 1, 6 5 524 95 100 100 100 100
adolescents (ages (179) (433) (308) (3,831) (1,069)
1-17 yrs)
Children and 720 0, 6 5 336 93 99 100
adolescents (ages (243) (253) (2,576)
2-18 yrs)
Adolescents (ages 1,440 0, 6 1 91 98 100
11-17 yrs) (294) (5,406)
Adults 1,440 0, 6 3 450 88 99 100
(293) (466) (4,383)
---------------------------------------------------------------------------------------------------------------------------------------------------------
* Source: SmithKline Beecham Biologicals.
+ ELISA units.
& 0 months represents timing of the initial dose and subsequent numbers represent months after the initial dose, except for infants, for whom
months indicate age at time of vaccination.
@ Geometric mean titer in milli-International Units per milliliter.
** GMT results are shown only for those time points at which data are available.
++ Without passively acquired maternal antibody.
=========================================================================================================================================================
Return to top. Table_7 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 7. Summary of selected studies on the immunogenicity of VAQTA, by age group *
============================================================================================================================================================
Age group (yrs) Dose + Schedule % Seroconversion (GMT @) by time after receiving first vaccine dose
(mos) & -----------------------------------------------------------------------------------
Persons (No.) 1 mo 6 mos 7 mos 12 mos 13 mos 18 mos 19 mos
------------------------------------------------------------------------------------------------------------------------------------------------------------
Children and adolescents 25 0, 6 132 98 97 100
(age 2-17 yrs) (37) (69) (6,755)
Children and adolescents 25 0, 12 133 100 100 95 100
(age 2-17 yrs) (46) (57) (52) (12,799)
Children and adolescents 25 0, 18 130 100 100 89 100
(age 2-17 yrs) (48) (252) (39) (9,792)
Adults 50 0, 6 1,436 95 98 100
(37) (210) (5,059)
------------------------------------------------------------------------------------------------------------------------------------------------------------
* Source: Merck & Company, Inc.
+ Units.
& 0 months represents timing of the initial dose and subsequent numbers represent months after the initial dose, except for infants, for whom
months indicate age at time of vaccination.
@ Geometric mean titer in milli-International Units per milliliter.
============================================================================================================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|