 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Use of Dietary Supplements Containing Folic Acid Among Women of Childbearing Age --- United States, 2005Neural tube defects (NTDs) are serious birth defects of the spine (spina bifida) and brain (anencephaly), affecting approximately 3,000 pregnancies each year in the United States (1). Daily periconceptional consumption of 400 µg of folic acid, as recommended by the Public Health Service (PHS) since 1992, reduces the occurrence of NTDs by 50%--70% (2). The Food and Drug Administration ordered mandatory fortification with folic acid of U.S. cereal grain products, beginning in 1998. However, despite a 26% reduction in NTDs, not all women of childbearing age receive adequate levels of folic acid from their diets (1). Therefore, increasing the number of women who take dietary supplements containing 400 µg of folic acid daily remains an important component of NTD prevention (3). This report summarizes results from the 2005 March of Dimes Gallup survey, which determined a decrease in the proportion of childbearing-aged women who reported taking folic acid in dietary supplements daily,* from 40% in 2004 to 33% in 2005, returning to a level consistent with that reported during 1995--2003 (Figure). These results emphasize the need for innovative programs to increase folic acid consumption to further reduce NTDs. The Gallup survey has been conducted since 1995 using a random-digit--dialed telephone interview of a proportionate stratified sample. Response rate for the 2005 survey was 32%, with 2,647 women aged 18--45 years responding; response rates for previous surveys ranged from 24% to 52%. Statistical estimates were weighted to reflect the total population of women aged 18--45 years in the contiguous United States who resided in households with telephones. The margin of error for estimates based on the total sample was +2%. To assess awareness of folic acid, respondents were asked, "Have you ever heard, read, or seen anything about folic acid?" To assess knowledge about folic acid, respondents were asked, "What have you heard, read, or seen about folic acid?" In addition, the survey asked questions regarding motivating factors and barriers to taking folic acid. In the 2005 survey, 33% of women of childbearing age reported taking folic acid daily, compared with 40% in 2004. This decrease was consistent across most demographic characteristics, with nonwhite, young, less educated, and lower-income women the least likely to report taking folic acid daily (Table 1). The percentage of women reporting awareness of folic acid increased from 78% in 2004 to 84% in 2005, an all-time high for the survey. However, the percentage of women knowing that folic acid prevents birth defects remained unchanged at 25%, and the percentage of women knowing that folic acid should be taken before pregnancy decreased from 12% in 2004 to 7% in 2005, the lowest percentage since 1997 (Figure). Twenty-six percent of women aged 18--45 years reported dieting during the preceding 6 months, relatively unchanged from 24% in 2004 (4). Of the women reporting dieting, 37% were taking folic acid daily and were nearly 30% more likely to be taking folic acid than nondieters (odds ratio [OR] = 1.27; 95% confidence interval [CI] = 1.13--1.41). Dieters were 50% more likely than nondieting women to believe that folic acid is important for women of childbearing age (OR = 1.50; CI = 1.34--1.68). Twenty-seven percent of dieting women reported being on low-carbohydrate diets, down substantially from 48% in 2004. Of the women on low-carbohydrate diets, 37% reported taking folic acid daily, down from 49% in 2004 (Table 2). In addition, 37% of women on other diets reported taking folic acid daily, down from 40% in 2004. Whereas in 2004 women on low-carbohydrate diets were 50% more likely to take folic acid daily than women on other diets, in 2005 folic acid consumption was similar among women in the two dieting groups (OR = 1.00; 95% CI = 0.81--1.24. Although in 2004, low-carbohydrate dieters were 30% more likely to believe that folic acid is important for women of childbearing age than women on other diets, in 2005, no difference was evident between women in these dieting groups (OR = 0.95; 95% CI = 0.76--1.18). Women were asked questions to determine why they do or do not take vitamin or mineral supplements. Women who did not report taking supplements were asked, "Why do you not take any vitamin or mineral supplements on a daily basis?" The most common barriers women noted were forgetting to take supplements (28%), perceiving they do not need them (16%), and believing they get needed nutrients and vitamins from food (9%). In 2005, to determine what might motivate women not taking vitamins or supplements to begin taking folic acid daily, respondents were asked, "For what specific need would you start taking a vitamin or mineral supplement?" The most common reported needs were being sick or in poor health (20%), a doctor's recommendation (20%), the need for energy (9%), being pregnant (8%), being deficient in any vitamins or minerals (7%), balancing the diet (6%), and keeping bones strong (6%). In addition, 11% cited no specific need that would motivate them to begin taking a vitamin or supplement. Among women who reported not consuming a vitamin or mineral supplement daily, 31% indicated they had received a doctor's recommendation. Older women were more likely to report receiving a doctor's recommendation (aged 35--45 years, 38%; 25--35 years, 38%; 18--24 years, 24%). Reported by: LLM Lindsey, PhD, CDC Foundation, Atlanta, Georgia. JR Petrini, PhD, March of Dimes Birth Defects Foundation, White Plains, New York. H Carter, MPH, C Prue, PhD, J Mulinare, MD, Div of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, CDC. Editorial Note:From 2004 to 2005, the percentage of women of childbearing age taking folic acid daily decreased from 40% to 33%. At least two possible explanations exist for this decline. First, 2004 survey results might have indicated a true increase in women's reported behavior that was not sustained; hence, in 2005, folic acid consumption returned to a level consistent with survey results since 1995. The reasons for such an increase in 2004 remain unclear. A second possible explanation is that the 2004 survey data were an anomaly and did not accurately reflect women's daily use of folic acid. The same sampling design and methodology have been used each year; however, multiple factors could have produced anomalous findings (e.g., nonrespresentative sampling or low response rates). The 2005 findings indicated that only 33% of women of childbearing age reported consuming folic acid daily. Data from NHANES indicate no change during 1991--2000 in reported consumption of 400 µg of folic acid daily among nonpregnant women aged 15--44 years (CDC, unpublished data, 2005). Given that reported folic acid consumption through supplementation has changed minimally during the preceding decade, new programs are needed. As one example, CDC is developing a program focused on ensuring that young women achieve optimal nutrition by encouraging that women in college consume a daily multivitamin containing 400 µg of folic acid. Fortification of food is a second approach demonstrated to reduce occurrence of NTDs (1). A recent report indicated a significant decrease in the prevalence of NTDs after implementation of folic acid fortification, reiterating the important role of fortification in reducing NTDs (5). Another report indicated an increase in blood folate levels for all segments of the U.S. population since fortification (6). Given the reported success of fortification and given that only one third of women report folic acid supplement use, another approach to reducing NTDs might be to develop programs that encourage women to consume folic acid from fortified foods. These foods, especially breakfast cereals, might be an easy alternative to ensure that women consume 100% of the recommended daily amount of folic acid every day. The reduction in the proportion of women who reported being on low-carbohydrate diets is consistent with other reports indicating a diminishing trend in use of low-carbohydrate diets (7). The slowing of this trend could facilitate NTD prevention because more women might be consuming foods fortified with folic acid (e.g., breads), which are the largest contributor of folate to diets in the United States (8). The findings in this report are subject to at least two limitations. First, the low response rate for this survey might have produced biased results. Second, because the majority of respondents to the survey were white, had at least some college, and had a median household income of approximately $50,000, the survey might not reflect the total U.S. population of women of childbearing age. With 67% of women not consuming a vitamin containing folic acid daily, innovative programs are needed to prevent NTDs. These programs must effectively address motivations and barriers regarding folic acid consumption to meet the national health objective for 2010 of increasing to 80% the proportion of women consuming 400 µg of folic acid daily (objective no. 16--16a) (9). References
* Women who reported taking a multivitamin, prenatal vitamin, or a folic-acid--only supplement in response to the question "What type of vitamin or mineral supplements do you take?" were coded as taking a vitamin containing folic acid (consistent with all previous surveys).
Table 1 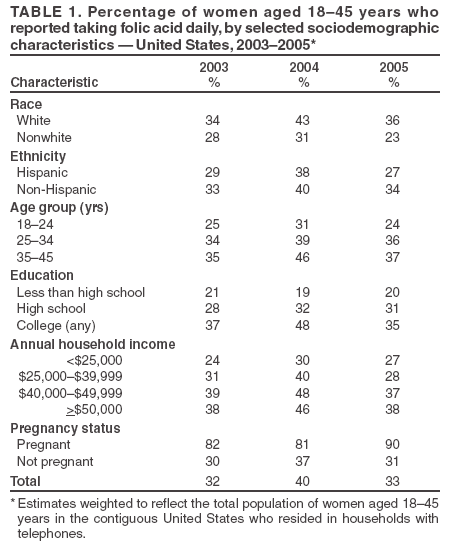 Return to top. Table 2 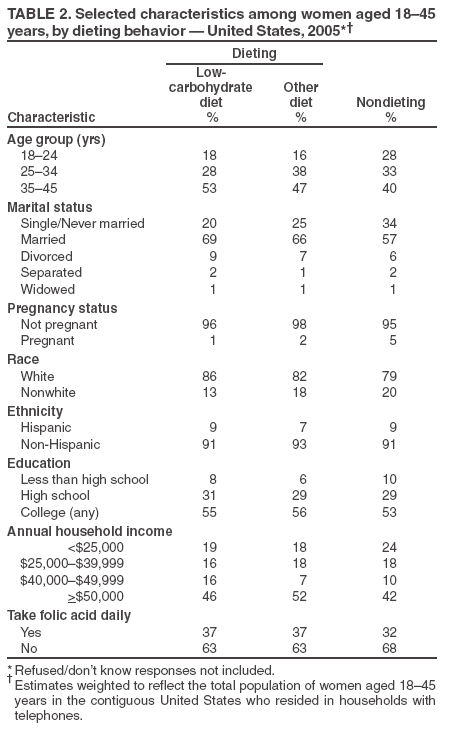 Return to top. Figure 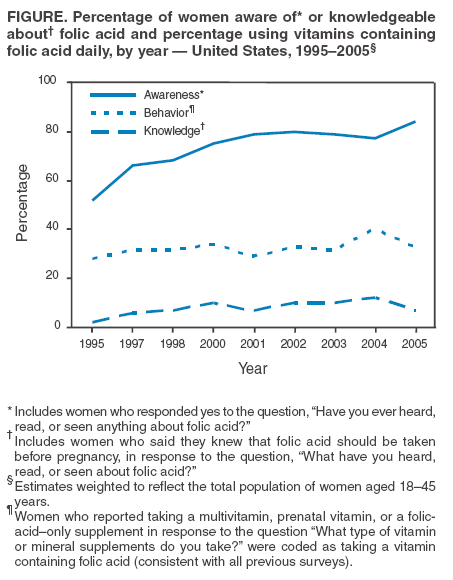 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 9/29/2005 |
|||||||||
|