 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Introduction of Routine HIV Testing in Prenatal Care --- Botswana, 2004In 2003, approximately 37% of pregnant women in Botswana (2001 population: 1.7 million; approximately 40,000 births per year) (1) were infected with human immunodeficiency virus (HIV) (2). Since 2001, all prenatal clinics in Botswana have offered HIV screening and interventions for prevention of mother-to-child transmission of HIV (PMTCT), which can decrease vertical transmission of HIV from 35%--40% to 5%--10% (3). Historically, HIV testing in Botswana has been performed after individual pretest counseling, with patients actively choosing whether to be tested (i.e., an "opt-in" approach). In 2003, 52% of pregnant women receiving prenatal care nationwide learned their HIV status. In 2004, to increase use of free national PMTCT and antiretroviral treatment (ARV) programs, Botswana began routine, noncompulsory (i.e., "opt-out") HIV screening in prenatal and other health-care settings. Concerns have been raised that routine testing in Africa might deter women from seeking prenatal care and might result in fewer women returning for their test results and HIV care after testing. To assess the early impact of routine testing on HIV-test acceptance and rates of return for care, the CDC Global AIDS Program and the PMTCT program in Botswana evaluated routine prenatal HIV testing at four clinics in Francistown, the second largest city in Botswana, where HIV prevalence has been >40% since 1995. This report describes the results of that assessment, which indicated that, during February--April 2004, the first 3 months of routine testing, 314 (90.5%) of 347 pregnant women were tested for HIV, compared with 381 (75.3%) of 506 women during October 2003--January 2004, the last 4 months of the opt-in testing period (p<0.001). However, many women who were tested never learned their HIV status because of logistical problems or not returning to the clinic. Substantial increases in HIV testing of pregnant women were also observed at the Francistown referral hospital and at prenatal clinics nationwide. These findings highlight the potential public health impact of routine HIV testing with rapid, same-day results for programs seeking to increase the number of persons with access to HIV-prevention and treatment services. Clinic EvaluationIn February 2004, in accordance with the new national policy of routine HIV testing in Botswana, personnel in four selected clinics were trained in a routine approach to prenatal HIV testing. Under the new system, existing PMTCT counselors (secondary-school graduates with 4 weeks of HIV-counseling training) held 10- to 15-minute group education sessions with pregnant women, using a flip chart as a discussion guide. The discussion focused on HIV transmission, PMTCT, ARV therapy, and testing needed for all mothers and infants. Women were informed that they would be routinely screened for HIV and other diseases. All were informed of their right to refuse testing. Women who did not want any of the tests were encouraged to discuss their concerns with the counselor. Women who arrived for prenatal care when no group could be convened received the same education individually. Women who did not refuse had blood drawn for HIV testing, which was performed offsite by laboratory technicians. Women usually received results and posttest counseling at their next scheduled prenatal visit (normally 1 month later). Women who were tested received individual posttest counseling, with a focus on PMTCT interventions for women who were identified as HIV positive, and were advised regarding next steps in medical care and psychosocial support. Data on prenatal-care attendance, HIV test acceptance, and receipt of HIV test results were collected from clinic logbooks for the 4 months before the routine testing project began and for the first 3 months of routine testing. The median number of women beginning prenatal care at all four clinics was 114 per month (range: 95--134 women) during the opt-in testing period and 130 (range: 97--154 women) during the routine testing period, with a total of 859 women beginning care during the period of data collection. Six women who were known to be HIV positive before their first prenatal visit were excluded from this analysis. The median time for HIV test results to return from the laboratory was 19 days (range: 0--59 days). Acceptance of HIV testing and receipt of test results increased (Figure) after the introduction of routine testing. However, no difference was observed in the percentage of women who were tested but did not receive results between the opt-in and routine periods (29.4% versus 33.0%; p=0.29). Of all 639 women for whom test results were available, 306 (47.9%) were HIV positive. Referral Hospital and National Program DataData from other sources also indicated an increase in the number of pregnant women learning their HIV status since routine testing began. Nyangabgwe Referral Hospital in Francistown is the site of approximately 10% of Botswana's annual deliveries, serving women from Francistown (including the four clinics involved in this project and eight other clinics where staff were trained in routine testing by project staff) and surrounding rural areas. For women who do not know their HIV status at delivery, routine testing is performed on the postnatal ward. Data from postnatal ward logbooks indicated that the percentage of women who delivered at Nyangabgwe Referral Hospital who knew their HIV status at the time of discharge increased from 50% in 2003 to 76% during the first 9 months of 2004. Data reported by all 24 health districts to the national PMTCT program indicated that the percentage of women who delivered in health facilities who knew their HIV status increased from 52% in 2003 to 69% during the first 6 months of 2004. As a complement to routine HIV testing, the government of Botswana plans to train HIV counselors in all health facilities to perform rapid, onsite HIV testing. This measure should reduce the number of clients who are tested but never receive results. Reported by: K Seipone, MD, Family Health Div, Botswana Ministry of Health; R Ntumy, MBChB, M Smith, MPH, BOTUSA Project, Gaborone; H Thuku, MD, Francistown District Health Team; L Mazhani, MD, Nyangabgwe Hospital, Francistown, Botswana. T Creek, MD, N Shaffer, MD, PH Kilmarx, MD, Global AIDS Program, National Center for HIV, STD, and TB Prevention, CDC. Editorial Note:Botswana has one of the greatest HIV burdens in the world. To improve coverage and effectiveness for its national PMTCT and ARV programs, Botswana recently adopted a national policy of routine HIV testing in prenatal and other health-care settings. The findings in this report demonstrate that group education and routine HIV testing were largely acceptable to this population of pregnant women in Botswana. Approximately 90% of women had an HIV test, and the introduction of routine testing did not lead to reductions in the number of women attending prenatal care or the percentage receiving test results compared with the opt-in period. Under both testing paradigms, many women who were tested did not learn their HIV status because laboratory testing was conducted offsite and results were not immediately available. Approximately 20% of women in Francistown never return to the clinic where they first seek prenatal care (Francistown District Health Team, unpublished data, 2002). Some women return but choose not to receive their results, and laboratory, clerical, and staffing difficulties add to the number of women who do not receive results during pregnancy. Interventions to prevent mother-to-child transmission of HIV are effective and safe (4), and HIV-infected women who know their status can also receive life-sustaining ARV therapy. Without intervention, 35%--40% of HIV-positive women transmit HIV to their infants; however, drug prophylaxis and formula feeding can reduce transmission to 5%--10%, and combination ARV therapy can reduce transmission to <1% (3). For these reasons, routine HIV testing has become the standard of care for pregnant women in developed countries (5), where HIV seroprevalence is relatively low. A routine approach to HIV testing has been rare in Africa, where HIV prevalence is higher, stigma associated with an HIV diagnosis has been a barrier to test acceptance, and large-scale PMTCT and ARV treatment programs are only recently becoming available. As part of worldwide efforts to expand access to PMTCT and ARV therapy, routine HIV testing of pregnant women (with the right to refuse) is recommended in the 2004 joint United Nations and World Health Organization policy statement on HIV testing (6). The findings in this report are subject to at least two limitations. First, this project involved clinics that had substantially higher-than-average testing acceptance even before implementation of the routine testing policy. Project clinics reported 76% acceptance at a time when the national program reported 52% acceptance; this was likely attributable to their highly committed staff. Second, data are being collected but are not yet available to determine whether women tested for HIV under the routine testing policy accept PMTCT interventions at the same rate as women tested under an opt-in testing policy. Introduction of routine HIV testing can improve HIV testing participation and access to prevention and treatment services in prenatal and other clinical settings. Use of same-day, rapid HIV testing can increase the impact of such a strategy in settings in which patients might not receive results from offsite testing. References
Figure 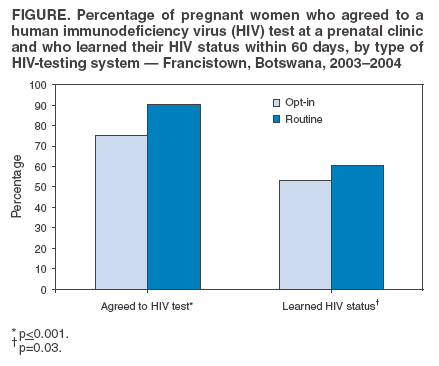 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/23/2004 |
|||||||||
This page last reviewed 11/23/2004
|