 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Adverse Events Associated with Anthrax Prophylaxis Among Postal Employees --- New Jersey, New York City, and the District of Columbia Metropolitan Area, 2001Antimicrobial prophylaxis to prevent inhalational anthrax has been recommended for persons potentially exposed to Bacillus anthracis as a result of the recent bioterrorist attacks (1). During October 26--November 6, 2001, an epidemiologic evaluation to detect adverse events associated with antimicrobial prophylaxis was conducted among 8,424 postal employees who had been offered antimicrobial prophylaxis for 60 days in New Jersey (NJ), New York City (NYC), and one postal facility in the District of Columbia (DC). This report summarizes preliminary results of that evaluation, which found that few employees receiving antimicrobial prophylaxis sought medical attention for symptoms that may have been associated with anaphylaxis. Persons with exposures to B. anthracis related to the bioterrorist attacks should complete the full 60-day course of antimicrobial prophylaxis. In NJ, NYC, and DC, a questionnaire was administered on days 7 to 10 after postal employees received prophylaxis (when they returned for medication refills). In NYC and DC, the questionnaire was self-administered by postal employees; in NJ, nurses interviewed postal workers and administered the questionnaire. Information was collected about the type of antimicrobial used, the occurrence of adverse events, medical attention sought for adverse events related to antimicrobial prophylaxis, and discontinuation of prophylaxis. Persons who reported hospitalization or sought medical attention for symptoms that may have been associated with anaphylaxis (i.e., difficulty breathing; throat tightness and difficulty swallowing; swelling of lips, tongue, or face; and rash, hives, and itchy skin) are being followed up closely by contacting patients and clinicians to confirm or exclude possible hospitalizations and life-threatening adverse events. Of the 8,424 postal employees offered antimicrobial prophylaxis, 5,819 (69%) completed or were administered the questionnaire to evaluate the occurrence of adverse events. A total of 3,863 (66%) had initiated antimicrobial prophylaxis*; of these, 3,428 (89%) reported using ciprofloxacin for antimicrobial prophylaxis; 435 (11%) used other antimicrobials (when ciprofloxacin was contraindicated), including doxycycline (6%) and amoxicillin (1%) (Table 1). Of the 3,428 persons on ciprofloxacin, 666 (19%) reported severe nausea, vomiting, diarrhea, or abdominal pain; 484 (14%) reported fainting, light-headedness, or dizziness; 250 (7%) reported heartburn or acid reflux; and 216 (6%) reported rashes, hives, or itchy skin. Of those persons taking ciprofloxacin, 287 (8%) discontinued the medication; 116 (3%) discontinued the medication because of adverse events, 27 (1%) discontinued because of fear of possible adverse events, and 28 (1%) stopped taking the drug because they "did not think it was needed." For the 3,863 persons on any medication for antimicrobial prophylaxis, 83 (2%) sought medical attention for symptoms that may have been associated with anaphylaxis. Among the 33 persons who sought medical attention for these symptoms in NJ and NYC, none was hospitalized and none of the symptoms was attributed to antimicrobial prophylaxis by clinicians who evaluated these persons. Follow-up of persons in DC who sought medical attention for symptoms that may have been associated with anaphylaxis is ongoing. Reported by: R Brechner, MD, State Epidemiologist, Maryland Dept of Health and Hygiene. G DiFerdinando, MD, E Bresnitz, MD, State Epidemiologist, New Jersey Dept of Health and Senior Svcs. New York City Dept of Health; SH Factor, MD, TD Matte, MD, Center for Urban Epidemiologic Studies, New York Academy of Medicine, New York. L Siegel, MD, S Adams, I Walks, MD, J Davies-Coles, PhD, M Richardson, MD, District of Columbia Dept of Health. E Peterson, MD, R Stroube, MD, State Epidemiologist, Virginia Dept of Health. National Center for Infectious Diseases; and EIS officers, CDC. Editorial Note:Among persons with exposures to B. anthracis related to the recent bioterrorist attacks, completion of a full 60-day course of antimicrobial prophylaxis is essential for preventing anthrax (1). Activities to promote adherence among postal employees in NJ, NYC, and DC include messages (e.g., posters at the worksite) to promote adherence, small group discussions with postal employees to identify and resolve barriers to adherence, and reminder devices (e.g., pocket calendars). In addition, a key component of promoting adherence is monitoring adverse events that might deter patients from taking antimicrobial prophylaxis. Information from these monitoring systems can be used to reassure workers of antimicrobial prophylaxis and to guide management of workers with potentially serious adverse events. Although adverse events were commonly reported by postal employees who participated in this evaluation and included gastrointestinal and dermatologic reactions, only 2% of persons surveyed sought medical care for symptoms that may have been associated with anaphylaxis. Overall rates of adverse events (regardless of attributability) in NJ, NYC, and DC are similar to the frequency of adverse events among other persons on antimicrobial prophylaxis for exposures to B. anthracis related to these bioterrorist attacks (2) and among persons on ciprofloxacin therapy for any indication (3,4). The higher rates of adverse events in NJ compared with NYC and DC (p=0.001), may be explained by the different mode of administration of the questionnaires (nurse versus self-administered). Discontinuation of therapy caused by adverse events was similar to other groups previously studied (5). Both active and passive monitoring of adverse events and promotion and assessment of adherence to prophylaxis will continue for the duration of the recommended postexposure prophylaxis. References
* The proportion of surveyed postal employees who had initiated prophylaxis varied across sites: 1,643 (99%) in DC, 434 (99%) in NJ, and 1,786 (48%) in NY. In NY, antimicrobial prophylaxis was recommended for approximately 1,800 postal employees who were at increased risk for anthrax and made available to another 2,600 postal employees at lower risk for anthrax. Table 1 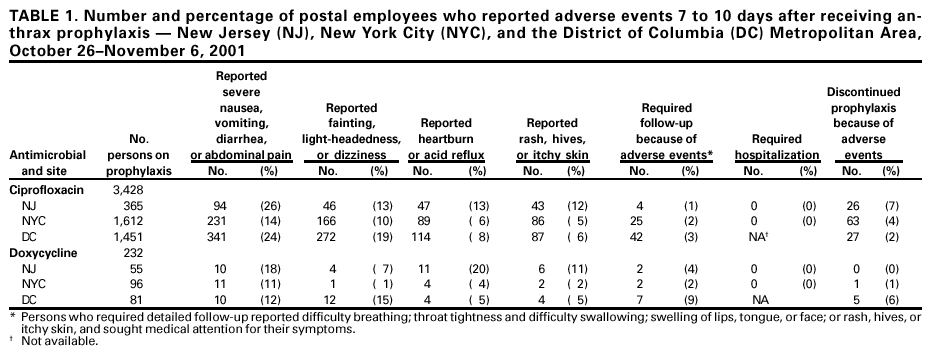 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/30/2001 |
|||||||||
This page last reviewed 11/30/2001
|