 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Influenza Activity --- United States, September 28, 2008--January 31, 2009From September 28, 2008, to January 31, 2009, influenza activity remained low in the United States but began to increase at the end of January. Thus far during the 2008--09 influenza season, influenza A viruses have predominated and are antigenically related to the 2008--09 influenza vaccine strains. Oseltamivir resistance has been detected in nearly all of the influenza A (H1N1) viruses tested so far during the 2008--09 season, with high levels of adamantane resistance among influenza A (H3N2) viruses. This report summarizes U.S. influenza activity* since the last update (1) and reviews interim recommendations for the use of influenza antiviral medications. Viral SurveillanceDuring September 28, 2008--January 31, 2009, approximately 150 World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System collaborating laboratories in the United States tested 81,842 respiratory specimens for influenza viruses; 4,336 (5.3%) were positive (Figure 1). Of these, 3,641 (84.0%) were influenza A viruses and 695 (16.0%) were influenza B viruses. Among the 3,641 influenza A viruses, 1,305 (35.8%) were subtyped; 1,135 (87.0%) were influenza A (H1), and 170 (13.0%) were influenza A (H3) viruses. Influenza virus--positive tests have been reported from 46 states and the District of Columbia in all nine of the surveillance regions since September 28, 2008. Antigenic CharacterizationWHO collaborating laboratories in the United States are requested to submit a subset of their influenza-positive respiratory specimens to CDC for further antigenic characterization. CDC has antigenically characterized 255 influenza viruses collected by U.S. laboratories during the 2008--09 season, including 142 influenza A (H1N1), 35 influenza A (H3N2), and 78 influenza B viruses. All influenza A (H1N1) and A (H3N2) viruses and 23 (29.5%) influenza B viruses were antigenically related to the components included in the 2008--09 influenza vaccine (A/Brisbane/59/2007-like [H1N1], A/Brisbane/10/2007-like [H3N2], and B/Florida/04/2006-like). The other 55 (70.5%) influenza B viruses belonged to the B/Victoria/02/87 lineage. Antiviral Resistance of Influenza Virus IsolatesCDC conducts surveillance for resistance of circulating influenza viruses to licensed antiviral medications: adamantanes (amantadine and rimantadine) and neuraminidase inhibitors (zanamivir and oseltamivir). Since October 1, 2008, 308 influenza viruses from 26 states have been tested for resistance to antiviral medications (Table 1). Of the 190 influenza A (H1N1) viruses tested, 185 (97.4%) were resistant to oseltamivir and all were susceptible to zanamivir. All 41 influenza A (H3N2) and all 77 influenza B viruses tested were susceptible to oseltamivir and zanamivir. Two influenza A (H1N1) viruses (1.1%) and all 41 influenza A (H3N2) viruses tested were resistant to adamantanes. None of the influenza A (H1N1) viruses tested were resistant to both oseltamivir and adamantanes. The adamantanes are not effective against influenza B viruses. CDC has solicited a representative sample of viruses from WHO collaborating laboratories in the United States for resistance testing throughout the season, and more specimens are expected as influenza activity increases. Novel Influenza A VirusesIn addition to the case reported from Texas in the previous update (1), one case of human infection with a novel influenza A virus was reported from South Dakota during the week ending January 31, 2009. A man aged 19 years was infected with swine influenza A (H1N1) virus in December 2008. The patient recovered fully. Investigation into swine exposure is ongoing. State-Specific Activity LevelsFor the week ending January 31, 2009, influenza activity† was reported as widespread in five states (Colorado, Delaware, New York, Texas, and Virginia) and regional in 21 others. Thirteen states and the District of Columbia reported local activity, and 11 states and Puerto Rico reported sporadic activity. Outpatient Illness SurveillanceSince September 28, 2008, the weekly percentage of outpatient visits for influenza-like illness (ILI)§ reported by approximately 1,500 U.S. sentinel providers comprising the U.S. Outpatient ILI Surveillance Network (ILINet), has ranged from 0.9% to 2.3%, which was reported during the most recent surveillance week (Figure 2). This is below the national baseline of 2.4% based on a 3-year average of noninfluenza weeks.¶ Four surveillance regions (East North Central, East South Central, New England, and West South Central) reported levels at or above their respective region-specific baselines. The five other surveillance regions reported percentages below their region-specific baselines. Pneumonia- and Influenza-Related MortalityFor the week ending January 31, 2009, pneumonia or influenza was reported as an underlying or contributing cause of death for 7.0% of all deaths reported to the 122 Cities Mortality Reporting System. This is below the epidemic threshold of 7.9% for that week. Since September 28, 2008, the weekly percentage of deaths attributed to pneumonia and influenza ranged from 6.0% to 7.5%, remaining below the epidemic threshold.** Influenza-Associated HospitalizationsHospitalizations associated with laboratory-confirmed influenza infections are monitored by two population-based surveillance networks, the Emerging Infections Program (EIP) and the New Vaccine Surveillance Network (NVSN). No influenza-associated pediatric hospitalizations have been reported in the NVSN this season. From October 31, 2008, to January 31, 2009, preliminary rates of laboratory-confirmed influenza-associated hospitalization reported by EIP for children aged 0--4 years and 5--17 years were 0.8 per 10,000 and 0.04 per 10,000, respectively. For adults aged 18--49 years, 50--64 years, and >65 years, the rates were 0.07, 0.1, and 0.3 per 10,000, respectively. Influenza-Related Pediatric MortalityThree influenza-associated pediatric deaths have been reported for the 2008--09 season. Two occurred during the week ending January 10, 2009 (reported from Colorado and Texas), and one during the week ending January 24, 2009 (reported from New York City). Two of the children had evidence of coinfection with Staphylococcus aureus, which was methicillin susceptible in one child and methicillin resistant in the other. Reported by: WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza. L Brammer, MPH, S Epperson, MPH, L Blanton, MPH, R Dhara, MPH, T Wallis, MS, L Finelli, DrPH, A Fiore, MD, L Gubavera, PhD, J Bresee, MD, A Klimov, PhD, N Cox, PhD, Influenza Div, National Center for Immunization and Respiratory Diseases; C Reed, DSc, EIS Officer, CDC. Editorial Note:From September 28, 2008, through January 31, 2009, the United States experienced low levels of influenza activity, but levels appeared to be increasing at the end of January. Activity is expected to increase throughout the country over the next few weeks. In 11 of the past 20 seasons, influenza activity has peaked during February or March (2). In response to increased oseltamivir resistance among circulating influenza A (H1N1) viruses detected through antiviral resistance testing early in the influenza season, on December 19, 2008 CDC issued interim guidelines for the use of influenza antiviral medications (3). Resistance patterns among circulating influenza virus types and subtypes have remained unchanged since that date. Providers are encouraged to review local or state influenza virus surveillance data to determine which types (A or B) and subtypes (H3N2 or H1N1) are circulating in their communities and to consider using diagnostic tests that can distinguish influenza A from influenza B. When influenza A (H1N1) virus infection or exposure is suspected, zanamivir or combination therapy with oseltamivir and rimantadine are more appropriate options than oseltamivir alone.†† Amantadine can be substituted for rimantadine in combination therapy. However, clinical experience with combination therapy is limited. Enhanced surveillance for oseltamivir-resistant viruses is ongoing at CDC, and clinicians should remain alert for changes in recommendations that might occur as the 2008--09 influenza season progresses. Vaccination remains the cornerstone of influenza prevention efforts. Influenza vaccination can prevent influenza virus infections from strains that are susceptible or resistant to antiviral medications. Thus far in the season, all influenza A (H1N1) viruses found to be oseltamivir resistant are antigenically similar to the components included in the 2008--09 vaccine. Vaccine is still available, and vaccination efforts should continue throughout the influenza season (which can persist as late as April or May) to protect as many persons from influenza and its complications as possible. Although influenza activity remains low nationwide, the first pediatric influenza-associated deaths of the 2008--09 season have been reported. Health-care providers should contact their local or state health department as soon as possible when deaths among children associated with laboratory-confirmed influenza are identified. Two deaths in children reported so far this season were associated with evidence of S. aureus coinfection. The proportion of pediatric deaths with evidence of S. aureus pneumonia or bacteremia increased substantially during the 2006--07 influenza season (4) and remained similarly high last season (CDC, unpublished data, 2008); and coinfection is known to occur in both children and adults. Health-care providers are encouraged to test persons hospitalized with respiratory illness for influenza, including those with suspected community-acquired pneumonia, so that appropriate antiviral treatment can be offered. In addition, providers should be alerted to the possibility of bacterial coinfection among persons with influenza, including both methicillin-susceptible and methicillin-resistant S. aureus coinfection, when choosing empiric antibiotic therapy for patients with suspected bacterial coinfection. Consensus guidelines for the management of community acquired pneumonia in adults, including influenza-associated pneumonia, were issued by The Infectious Disease Society of America and the American Thoracic Society in 2007 (5). Two cases of human infection with swine influenza have been reported so far this season. Although human infection with swine influenza is uncommon, sporadic cases have occurred in past years, usually among persons in direct contact with ill pigs or who have been in places where pigs might have been present (e.g., agricultural fairs and farms). Sporadic cases of human infections with swine influenza viruses identified in recent years have not resulted in sustained human-to-human transmission or community outbreaks. Nonetheless, when cases are identified, CDC recommends thorough investigations to evaluate the extent of the outbreak and possible human-to-human transmission, because transmission patterns can change with changes in swine influenza viruses. CDC continues to conduct surveillance to provide up-to-date recommendations regarding prevention and treatment of influenza. Influenza surveillance reports for the United States are posted online weekly during October--May and are available at http://www.cdc.gov/flu/weekly/fluactivity.htm. Additional information regarding influenza viruses, influenza surveillance, influenza vaccine, and avian influenza is available at http://www.cdc.gov/flu. Acknowledgments This report is based, in part, on data contributed by participating state and territorial health departments and state public health laboratories, WHO collaborating laboratories, National Respiratory and Enteric Virus Surveillance System collaborating laboratories, the U.S. Influenza Sentinel Provider Surveillance System, and the 122 Cities Mortality Reporting System.
References
* The CDC influenza surveillance system collects five categories of information from 10 data sources: 1) viral surveillance (U.S. World Health Organization collaborating laboratories, the National Respiratory and Enteric Virus Surveillance System, and novel influenza A virus case reporting), 2) outpatient illness surveillance (U.S. Influenza Sentinel Provider Surveillance Network and the U.S. Department of Veterans Affairs/U.S. Department of Defense BioSense Outpatient Surveillance System), 3) mortality (122 Cities Mortality Reporting System and influenza-associated pediatric mortality reports), 4) hospitalizations (Emerging Infections Program and New Vaccine Surveillance Network), and 5) summary of geographic spread of influenza (state and territorial epidemiologist reports). † The five levels of activity are 1) no activity; 2) sporadic: isolated laboratory-confirmed influenza cases or a laboratory-confirmed outbreak in one institution, with no increase in activity; 3) local: increased influenza-like illness (ILI), or at least two institutional outbreaks (ILI or laboratory-confirmed influenza) in one region with recent laboratory evidence of influenza in that region, and virus activity no greater than sporadic in other regions; 4) regional: increased ILI activity or institutional outbreaks (ILI or laboratory-confirmed influenza) in at least two but less than half of the regions in the state with recent laboratory evidence of influenza in those regions; and 5) widespread: increased ILI activity or institutional outbreaks (ILI or laboratory-confirmed influenza) in at least half the regions in the state with recent laboratory evidence of influenza in the state. § Defined as a temperature of >100.0°F (>37.8°C), oral or equivalent, and cough and/or sore throat, in the absence of a known cause other than influenza. ¶ The national and regional baselines are the mean percentage of visits for ILI during noninfluenza weeks for the previous three seasons plus two standard deviations. A noninfluenza week is a week during which <10% of specimens tested positive for influenza. National and regional percentages of patient visits for ILI are weighted on the basis of state population. Use of the national baseline for regional data is not appropriate. ** The seasonal baseline proportion of pneumonia and influenza deaths is projected using a robust regression procedure in which a periodic regression model is applied to the observed percentage of deaths from pneumonia and influenza that were reported by the 122 Cities Mortality Reporting System during the preceding 5 years. The epidemic threshold is 1.645 standard deviations above the seasonal baseline. †† Available at http://www.cdc.gov/flu/professionals/antivirals/index.htm. Table 1 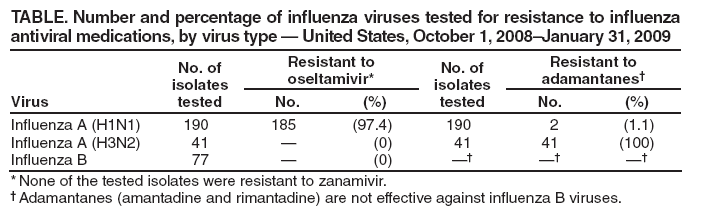 Return to top. Figure 1 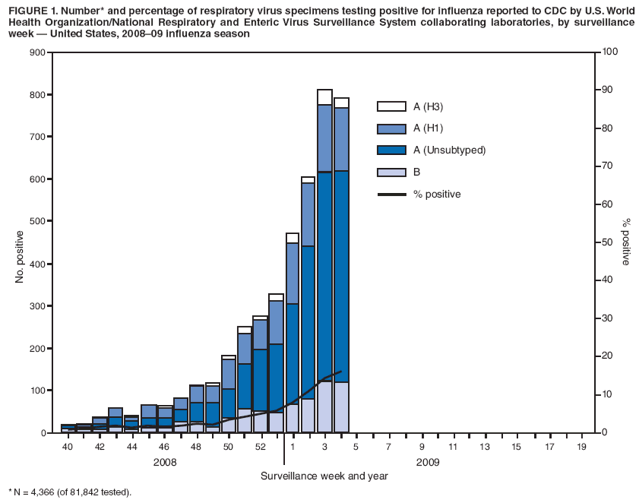 Return to top. Figure 2 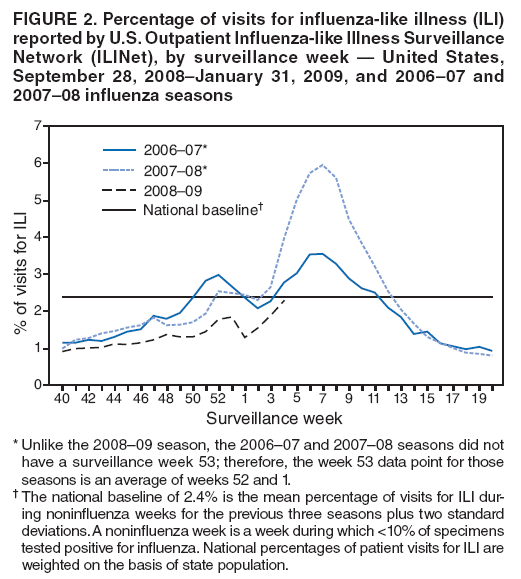 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 2/12/2009 |
|||||||||
|