 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Hepatitis B Vaccination for Injection Drug Users --- Pierce County, Washington, 2000Hepatitis B vaccination has been recommended for injection drug users (IDUs) since 1982, but vaccination coverage of IDUs remains low (1), and outbreaks of hepatitis B among IDUs continue to occur. An outbreak of hepatitis B primarily among IDUs in Pierce County, Washington, detected in April 2000, included 60 cases and resulted in three deaths among IDUs co-infected with hepatitis delta virus. A program to administer hepatitis B vaccine to IDUs was implemented to control the outbreak, and the number of cases identified decreased from 13 per month in May to two cases since November. This report describes a vaccination program during which IDUs accepted hepatitis B vaccination provided free of charge in community-based settings and illustrates how effective hepatitis B vaccination programs targeted at IDUs can be implemented through collaborations between departments of health and corrections and community organizations. Because the first seven identified case-patients used the local syringe exchange program, hepatitis B vaccination clinics were established in May 2000 at syringe exchange sites and the county health department. Later, vaccination clinics were added at other sites, including the county jail, a soup kitchen, and a substance abuse treatment program for women. Community outreach workers, syringe exchange and methadone clinic staff, and a local media campaign promoted hepatitis B vaccination at these clinics. Hepatitis B vaccine, provided by the Washington State Department of Health, was offered at no charge and administered on a 0-, 1-, and 4-month schedule by the county health department. Participants received a $5 reimbursement for travel with each vaccine dose. At the time the first vaccine dose was administered, recipients were tested for previous hepatitis B virus (HBV) infection using total antibody to hepatitis B core antigen (anti-HBc), and informed of the results when they returned for the second dose. Persons susceptible to HBV infection (total anti-HBc negative) or from whom a serum sample could not be obtained were advised to complete the vaccination series. Participants were given vaccination cards listing vaccine doses received and dates for subsequent doses. A standard questionnaire administered by trained interviewers was used to collect demographic and risk behavior information from persons attending the vaccination clinics during May--July 2000. Rates of vaccination series (3 dose) completion as of January 2001 were calculated for persons who initiated vaccination during May--July. During May--December, a total of 1981 persons initiated hepatitis B vaccination. The median age of vaccinated persons was 39 years (range: 16--77 years). Overall, 1205 participants (60.8%) reported ever injecting drugs. Of the 874 persons who completed the questionnaire and identified themselves as IDUs, 603 (69%) reported obtaining most of their syringes from the syringe exchange program. Of the 390 persons who completed the questionnaire and did not report a history of injection drug use, 287 (74%) reported other risk factors for HBV infection (e.g., sex with an IDU, multiple sex partners, being a man who has sex with men, or engaging in commercial sex work). Of the 1733 persons who underwent prevaccination testing, 708 (40%) had serologic evidence of previous HBV infection, including 518 (51%) of 1014 IDUs tested and 111 (20%) of 549 persons tested who reported not injecting drugs (Table 1). As of January 2001, 673 (53%) of 1261 persons due for the second dose of vaccine and 216 (27%) of 813 persons due for the third dose had received it. Of the 683 IDUs who needed to complete the vaccine series, 372 (55%) of those due for the second dose and 130 (27%) of those due for the third dose received it. The vaccination series completion rate among IDUs (27%) was similar to that of non-IDUs (28%). Of the 1981 persons who initiated the hepatitis B vaccination series, 1051 (53%) received the first dose at the syringe exchange, 301 (15%) at the county jail while incarcerated, 174 (9%) at the health department, 315 (16%) at the soup kitchen, and 138 (7%) at other community sites (Table 1). Most persons (77%) received their second vaccine dose at the site where they initiated vaccination, including those who initiated the series in jail (82%). Reasons for accepting vaccination were reported by 688 IDUs who completed the questionnaire. Reasons included knowing that persons were dying from hepatitis (24%), wanting to get vaccinated (17%), needing the financial reimbursement (15%), wanting to be tested for hepatitis B (14%), having received advice from syringe exchange staff (13%) or friends (10%), and other reasons (6%). Reported by: D Purchase, Point Defiance AIDS Projects; K Mottram, C Miron, D Sharma, F Cruz-Uribe, MD, Tacoma Pierce County Health Dept, Tacoma; S McInelly, J Kobayashi, MD, State Communicable Disease Epidemiologist, Washington State Dept of Health. Div of Viral Hepatitis, National Center for Infectious Diseases; and an EIS Officer, CDC. Editorial Note:This report describes the successful implementation of a hepatitis B vaccination program for IDUs and other high-risk persons that involved public health departments and community-based organizations and provided hepatitis B vaccine to approximately 1900 persons. Assuming that half of the estimated 6000 IDUs residing in Pierce County (K. Mottram, Tacoma Pierce County Health Department, personal communication, 2001) are susceptible to HBV infection, at least 20% of this population received one or more doses of vaccine during the first 8 months of the program. Although no efforts besides providing a vaccination card were made to remind participants to return for subsequent doses, approximately half of susceptible IDUs received a second vaccine dose. This second dose completion rate was similar to that reported among persons offered hepatitis B vaccination at a sexually transmitted disease (STD) clinic (2) and higher than that reported among IDUs offered vaccination at community-based sites that did not include a needle exchange program (3). The vaccination program in Pierce County is ongoing, and completion rates are expected to increase. Although completion of the hepatitis B vaccination series is desirable, protective levels of antibody develop in 30% of adults after one dose and in 89% after two doses (4). Therefore, a substantial proportion of IDUs who have not completed the vaccination series probably are protected against HBV infection. Completion of the vaccination series should not be considered a prerequisite for initiating vaccination in high-risk persons. Vaccination of IDUs to prevent HBV infection presents substantial challenges. Approximately 70% of IDUs are infected within 5 years of initiating injection drug use (5), and prevention of HBV infections in this risk group requires vaccination soon after the initiation of risk-taking behaviors. However, many IDUs lack health insurance or a regular source of medical care and receive care in settings where vaccination is not routinely offered (e.g., emergency departments) (6). In addition, many medical providers do not ascertain a history of injection drug use or offer hepatitis B vaccination to IDUs (7). Relatively high vaccination coverage levels can be achieved among IDUs participating in harm reduction services such as syringe exchange programs. For example, pneumococcal and influenza vaccination was accepted by 86% of IDUs at a syringe exchange program in New York City (5). Offering hepatitis B vaccine at nontraditional sites such as syringe exchange programs and jails (3) and providing modest monetary incentives (8) can increase hepatitis B vaccination coverage among IDUs. Although the outbreak may have increased concern among IDUs about the risks for HBV infection, approximately 75% of IDUs who attended the vaccination clinics reported reasons other than awareness of the outbreak as their motivation for getting vaccinated. This finding suggests that hepatitis B vaccination for IDUs also could be successfully incorporated into syringe exchange programs in nonoutbreak settings. The findings in this report are subject to at least two limitations. First, because the data on drug-use practices were self-reported, they may be inaccurate and the proportion of IDUs that attended the vaccination clinics may be underestimated. Second, most IDUs who were vaccinated participated in a syringe exchange program. The results of this program may not be generalizable in settings that lack such access to IDUs. This report illustrates how hepatitis B vaccination programs targeted at IDUs can be implemented through collaborations between departments of health and corrections and community organizations and demonstrates the feasibility of vaccinating IDUs in various community settings. National programs to provide hepatitis B vaccine to high-risk persons are needed to apply these findings widely. References
Table 1 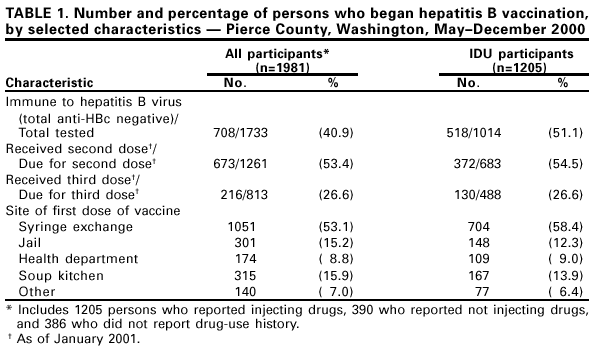 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 5/17/2001 |
|||||||||
This page last reviewed 5/17/2001
|