 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Effectiveness of a Middle School Vaccination Law --- California, 1999--2001In 1996, the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, the American Association of Family Physicians, and the American Medical Association recommended a routine health-care visit for adolescents aged 11--12 years (1). During this visit, adolescents not fully vaccinated should receive up to four recommended vaccines (hepatitis B, a measles-containing vaccine [MCV], varicella, and tetanus-diphtheria) and other preventive services and counseling. Because many adolescents are not up-to-date for all of these vaccines, 43 states have developed middle school entrance requirements or laws for one or more of these vaccines. Since 1997, CDC, in collaboration with the Pre-Teen Health Project in San Diego, California, has evaluated the impact of the state's middle school vaccination law, which requires students entering seventh grade on or after July 1, 1999, to have documented receipt of three doses of hepatitis B and two doses of MCV or to have obtained a written exemption based on personal beliefs or medical grounds. This report summarizes the results of that analysis, which indicate that when school entrance requirements are enforced, high vaccination coverage can be achieved. During the 1999--2000 school year, the law affected 464,476 seventh-grade students in California, including 38,875 in San Diego County. For this analysis, three different surveys were used to assess the impact of changes in the vaccination law. First, to estimate baseline coverage, a countywide telephone random-digit--dialed vaccination coverage survey of fifth and sixth graders was conducted during April--June 1998 in San Diego County (2). Second, to evaluate compliance with state school vaccination requirements, California requires each school to report coverage as of October of each year, based on records obtained for every enrolled student. Finally, health-care officials confirm these results by reviewing vaccination records in randomly selected schools statewide during February--April (3). During the 1999--2000 and 2000--2001 school years, 199 and 163 schools, respectively, had their vaccination records validated statewide. In the 1998 baseline telephone survey of 741 households with adolescents in San Diego County, vaccination history was verified through the parent-held records of 203 fifth and sixth graders (2). Of these, 142 (70.0%) had received two doses of MCV, 32 (15.8%) had received three doses of hepatitis B, and 27 (13.3%) had received both vaccines. During October 1999, data from all 315 San Diego County schools with seventh-grade students (38,875 seventh graders) indicated that 36,005 (92.6%) students had received two doses of MCV, and 26,614 (68.5%) had received three doses of hepatitis B vaccine. Overall, 26,110 (67.2%) students were in compliance with the law by vaccination and 691 (1.8%) by exemption. Of 12,074 adolescents not in compliance,10,814 (89.6%) were in the process of completing the three-dose hepatitis B series. Coverage continued to increase through the end of the school year as unvaccinated students completed the three-dose hepatitis B series. Similar coverage levels were achieved statewide during October 1999 and increased by the time of the review during February--April 2000 (Table 1). In October 2000, the beginning of the second year the law was in effect, coverage was higher than in October 1999 (Table 1). Reported by: K Gustafson, W Wang, S Ross, County of San Diego Health and Human Svcs Agency; L Linton, San Diego State Univ Graduate School of Public Health, San Diego; N Smith, N Gandhi, Immunization Br, California Dept of Health Svcs. Health Svcs Research and Evaluation Br, Immunization Svcs Div, National Immunization Program, CDC. Editorial Note:As of July 2001, of the 43 states with middle school vaccination laws, 27 required students entering middle school to be fully vaccinated against hepatitis B, and 41 required students to have received two doses of MCV. The findings in this report indicate that school vaccination laws are an important strategy for promoting universal coverage with hepatitis B and MCV among an adolescent population. Although the passage of a vaccination law is an important step in increasing coverage, cooperation by the public health community in enforcing the law is essential for successful implementation (4). San Diego County achieved a high level of coverage through monitoring and close cooperation with schools, frequent reminders to parents, and exclusion of students from school when necessary. The 1991 recommendation for universal infant vaccination with hepatitis B vaccine and state requirements for proof of vaccination at kindergarten entry produced a cohort of children in the United States who are highly vaccinated against hepatitis B. However, in 1998, when only eight states had hepatitis B vaccination coverage laws for middle school students, national coverage for hepatitis B vaccine among persons aged 13--15 years with a vaccination record was an estimated 27.3% (CDC, unpublished data, 2001). Even among adolescents enrolled in prepaid health-care plans, coverage remains low in the absence of a law (5). A statewide evaluation of a middle school vaccination law in Florida indicated that, following implementation of changes to the Florida Administrative Code requiring adolescent vaccinations, 61.8% of students were vaccinated fully with three doses of hepatitis B within 3 months of the start of the 1997 school year (6). However, no mechanism was in place in Florida to determine the number of students that had completed the series of three doses before or after that time in the school year. The success of voluntary hepatitis B vaccination programs does not necessarily predict sustainable large-scale implementation. In a pilot program in San Diego County during 1993--1995, 61% of fourth through ninth graders in 16 schools in San Diego County were vaccinated (7). However, by 1998, countywide coverage was only 15.8% among fifth and sixth graders (2). Hepatitis B vaccination is especially important for adolescents because approximately 9% of hepatitis B occurs in adolescents and an additional 45% in persons aged 20--29 years (8; CDC, unpublished data, 2001). Adolescents also should be up-to-date with two doses of MCV because interruption of measles transmission in the United States during the 1990s was a result of increased coverage and the administration of a second dose of MCV to children and adolescents (9). The findings in this report are subject to at least three limitations. First, the findings are subject to the effect of confounding because it was not possible to assess changes in coverage among seventh graders that would have occurred in the absence of a law. Second, because three methods were used to assess coverage (random-digit--dialing, school reporting, and on-site record reviews), results may differ from those found if the same method was used at each point in time. Finally, only confirmed vaccination histories were used in the telephone survey, and most parents surveyed could not find their child's vaccination record. In California and Florida, the two states in which middle school vaccination requirements have been evaluated, the laws resulted in a substantial increase in hepatitis B vaccination coverage and, in California, high second dose MCV coverage (6). The effectiveness of the California law is consistent with evaluations of vaccinations required for school entry in other age groups, suggesting that vaccination requirements and laws are an effective means of protecting young persons in all age groups from vaccine preventable diseases (4). References*
*All MMWR references are available on the Internet at <http://www.cdc.gov/mmwr>. Use the search function to find specific articles. Table 1 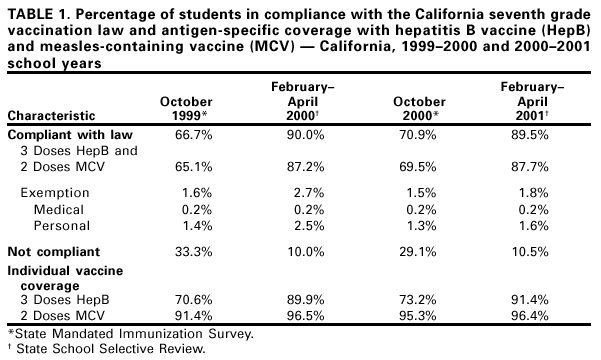 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 8/10/2001 |
|||||||||
This page last reviewed 8/10/2001
|