 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Surveillance for Waterborne Disease and Outbreaks Associated with Recreational Water --- United States, 2003--2004Eric J. Dziuban1,2
Corresponding author: Corresponding author: Michael J. Beach, PhD, Division of Parasitic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases (proposed), 4770 Buford Hwy., NE, MS F-22, Atlanta, GA 30341. Telephone: 770-488-7763; Fax: 770-488-7761; E-mail: mbeach@cdc.gov.
AbstractProblem/Condition: Since 1971, CDC, the U.S. Environmental Protection Agency, and the Council of State and Territorial Epidemiologists have collaboratively maintained the Waterborne Disease and Outbreak Surveillance System for collecting and reporting waterborne disease and outbreak (WBDO)-related data. In 1978, WBDOs associated with recreational water (natural and treated water) were added. This system is the primary source of data regarding the scope and effects of WBDOs in the United States. Reporting Period: Data presented summarize WBDOs associated with recreational water that occurred during January 2003--December 2004 and one previously unreported outbreak from 2002. Description of the System: Public health departments in the states, territories, localities, and the Freely Associated States (i.e., the Republic of the Marshall Islands, the Federated States of Micronesia, and the Republic of Palau, formerly parts of the U.S.-administered Trust Territory of the Pacific Islands) have primary responsibility for detecting, investigating, and voluntarily reporting WBDOs to CDC. Although the surveillance system includes data for WBDOs associated with drinking water, recreational water, and water not intended for drinking, only cases and outbreaks associated with recreational water are summarized in this report. Results: During 2003--2004, a total 62 WBDOs associated with recreational water were reported by 26 states and Guam. Illness occurred in 2,698 persons, resulting in 58 hospitalizations and one death. The median outbreak size was 14 persons (range: 1--617 persons). Of the 62 WBDOs, 30 (48.4%) were outbreaks of gastroenteritis that resulted from infectious agents, chemicals, or toxins; 13 (21.0%) were outbreaks of dermatitis; and seven (11.3%) were outbreaks of acute respiratory illness (ARI). The remaining 12 WBDOs resulted in primary amebic meningoencephalitis (n = one), meningitis (n = one), leptospirosis (n = one), otitis externa (n = one), and mixed illnesses (n = eight). WBDOs associated with gastroenteritis resulted in 1,945 (72.1%) of 2,698 illnesses. Forty-three (69.4%) WBDOs occurred at treated water venues, resulting in 2,446 (90.7%) cases of illness. The etiologic agent was confirmed in 44 (71.0%) of the 62 WBDOs, suspected in 15 (24.2%), and unidentified in three (4.8%). Twenty (32.3%) WBDOs had a bacterial etiology; 15 (24.2%), parasitic; six (9.7%), viral; and three (4.8%), chemical or toxin. Among the 30 gastroenteritis outbreaks, Cryptosporidium was confirmed as the causal agent in 11 (36.7%), and all except one of these outbreaks occurred in treated water venues where Cryptosporidium caused 55.6% (10/18) of the gastroenteritis outbreaks. In this report, 142 Vibrio illnesses (reported to the Cholera and Other Vibrio Illness Surveillance System) that were associated with recreational water exposure were analyzed separately. The most commonly reported species were Vibrio vulnificus, V. alginolyticus, and V. parahaemolyticus. V. vulnificus illnesses associated with recreational water exposure had the highest Vibrio illness hospitalization (87.2%) and mortality (12.8%) rates. Interpretation: The number of WBDOs summarized in this report and the trends in recreational water-associated disease and outbreaks are consistent with previous years. Outbreaks, especially the largest ones, are most likely to be associated with summer months, treated water venues, and gastrointestinal illness. Approximately 60% of illnesses reported for 2003--2004 were associated with the seven largest outbreaks (>100 cases). Deficiencies leading to WBDOs included problems with water quality, venue design, usage, and maintenance. Public Health Actions: CDC uses WBDO surveillance data to 1) identify the etiologic agents, types of aquatic venues, water-treatment systems, and deficiencies associated with outbreaks; 2) evaluate the adequacy of efforts (i.e., regulations and public awareness activities) to provide safe recreational water; and 3) establish public health prevention priorities that might lead to improved regulations and prevention measures at the local, state, and federal levels. IntroductionDuring 1920--1970, statistical data regarding waterborne disease and outbreaks (WBDOs) in the United States were collected by different researchers and federal agencies (1). Since 1971, CDC, the U.S. Environmental Protection Agency (EPA), and the Council of State and Territorial Epidemiologists (CSTE) have collaboratively maintained the Waterborne Disease and Outbreak Surveillance System (WBDOSS), a surveillance system that tracks the occurrences and causes of WBDOs associated with drinking water (2--11). In 1978, WBDOs associated with recreational water were added to the surveillance system. The types of outbreaks and diseases included in the surveillance summaries have expanded multiple times. Outbreaks of Pontiac fever (PF) were added in 1989 (9), outbreaks of Legionnaires' disease were added in 2001 (2), and single cases of Vibrio illness associated with recreational water but not limited to preexisting wounds have been added in this report. WBDOs associated with drinking water and water not intended for drinking are presented in a separate report (12). WBDO surveillance activities are intended to 1) characterize the epidemiology of WBDOs; 2) identify changing trends in the etiologic agents and other risk factors associated with WBDOs; 3) identify major deficiencies in providing safe recreational water; 4) encourage public health personnel to detect and investigate WBDOs; and 5) foster collaboration among local, state, federal, and international agencies on initiatives to prevent waterborne disease. Data obtained through the WBDOSS are useful for identifying major deficiencies in providing safe recreational water, influencing research priorities, supporting public health recommendations, and encouraging improved water-quality policies and regulations. However, the WBDOs summarized in this report represent only a portion of the illness associated with water exposure. The surveillance information described in this report does not include endemic waterborne disease risks, although studies to measure the levels of endemic illness associated with recreational water use are needed. Reliable estimates of the number of unrecognized WBDOs are not available. BackgroundRegulation of Recreational Water QualityRecreational water use has involved a risk for disease for virtually all of human history. Evidence of schistosomiasis, a parasitic disease only contracted by having contact with contaminated water, can be found in Egyptian mummies approximately 3,000 years old (13). In the United States, state and local governments establish and enforce regulations for protecting recreational water from naturally occurring or human-made contaminants. For treated water venues (e.g., swimming and wading pools), no federal regulatory agency or national guidelines for standards of operation, disinfection, or filtration exist. Because these swimming pool codes are developed and enforced by state and local health departments, substantial variation is observed across the country in terms of policy, compliance, and enforcement (14). In 1986, EPA published bacterial water-quality criteria for untreated fresh and marine water sources (15) and made these criteria water-quality standards for the states and territories that did not adopt the criteria before 2004. For freshwater (e.g., lakes and rivers), EPA has recommended criteria that the monthly geometric mean water-quality indicator concentration be <33 CFU/100 mL for enterococci or <126 CFU/100 mL for Escherichia coli. For marine water, EPA has recommended criteria that the monthly geometric mean water-quality indicator concentration be <35 CFU/100 mL for enterococci. However, state and local authorities have discretionary authority to determine which interventions should be used (e.g., posting signs to alert visitors of water contamination or closing the beach for swimming) when these limits have been exceeded. Natural processes to improve the quality of untreated recreational water might take days to months to occur. In contrast, disinfection, filtration, and pool drainage are examples of techniques that are used to restore a safe swimming environment in treated water venues. Whereas pools and other treated-water venues might need to be closed for a period of time and continued monitoring might be necessary, decontamination is usually feasible in hours to days. EPA's Action Plan for Beaches and Recreational Waters (i.e., Beach Watch) was developed as part of the Clean Water Action Plan (available at http://www.cleanwater.gov). The intent of Beach Watch is to assist state, tribal, and local authorities in strengthening and extending existing programs to protect users of fresh and marine recreational waters. As part of the Beaches Act of 2000, the U.S. Congress directed EPA to create a new set of guidelines for recreational water based on novel water-quality indicators. As a result, EPA has been collaborating with CDC since 2002 on a series of epidemiologic studies at fresh and marine water recreational beaches in the United States. Information on the National Epidemiologic and Environmental Assessment of Recreational (NEEAR) Water Study is available at http://www.epa.gov/nerlcwww/neearnerl.htm. This study is being conducted to test rapid new water-quality methods that are able to produce results in <2 hours and to correlate these indicators with health effects among beachgoers. Preliminary results from two Great Lakes beaches have demonstrated an association between an increasing signal detected by a quantitative polymerase chain reaction--based test method for enterococci and human health effects (16). MethodsData SourcesPublic health departments in individual states, territories, localities, and the Freely Associated States (FAS)* have the primary responsibility for detection and investigation of WBDOs. The outbreaks are voluntarily reported to CDC through a standard form (i.e., CDC form 52.12) available at http://www.cdc.gov/healthyswimming/downloads/cdc_5212_waterborne.pdf. The form solicits data on the WBDO, including characteristics of person, place, time, and results of epidemiologic studies, disease symptoms, microbiology, and water sampling. Information regarding the setting of the outbreak also is gathered, including water-supply descriptions, any sanitary measures in place, and possible factors contributing to the contamination of the water. Public health professionals in each state or locality are designated as WBDO surveillance coordinators, and CDC annually requests reports from each coordinator and conducts as much follow-up correspondence as needed to resolve unaddressed questions. Contact is made with all states or localities, including those without WBDO reports. Information is sometimes solicited from other CDC surveillance systems and confirmed with the state or locality for inclusion as a WBDO. Outbreaks or cases, where applicable, are assigned to a state, based on location of exposure rather than state of residence of ill persons. Numeric and text data from the CDC form and any supporting documentation are entered into a database for analysis. Although all WBDOs are collected through the same CDC reporting system, the recreational water-associated outbreaks are analyzed and published in this report separately from drinking water-associated outbreaks and other WBDOs (12). SAS programming is used for all statistical analyses. Definitions†The unit of analysis for the WBDOSS is typically an outbreak, not an individual case of a waterborne disease. To be defined as a WBDO associated with recreational water, an event must meet two criteria. First, two or more persons must be epidemiologically linked by location of exposure to water, time, and illness. This criterion is waived for 1) single cases of laboratory-confirmed primary amebic meningoencephalitis (PAM) associated with recreational water, 2) wound infections or other Vibrio infections associated with recreational water, and 3) single cases of chemical/toxin poisoning if water or air-quality data indicate contamination by the chemical/toxin. Second, the epidemiologic evidence must implicate water as the probable source of the illness. WBDOs associated with cruise ships are not summarized in this report. Recreational water settings include swimming pools, wading pools, spas, waterslides, interactive fountains, wet decks, and fresh and marine bodies of water. For this analysis, the WBDOs are separated by venue as untreated (i.e., fresh and marine surface water) or treated (i.e., disinfected [e.g., chlorinated]) water. Strength of Evidence Classification for Waterborne Disease and OutbreaksWBDOs reported to the WBDOSS are classified according to the strength of evidence that implicates water as the vehicle of transmission (Table 1). The classification scheme (i.e., Classes I--IV) is based on the epidemiologic and water-quality data provided on the WBDO report form. Although in certain instances WBDOs without water-quality data were included in this report, outbreaks that lacked epidemiologic data linking the outbreak to water were excluded. Class I indicates that adequate epidemiologic and water-quality data were reported (Table 1). However, the classification does not necessarily imply that an investigation was optimally conducted nor does a classification of II, III, or IV imply that an investigation was inadequate or incomplete. Outbreaks and the resulting investigations occur under different circumstances, and not all outbreaks can or should be rigorously investigated. In addition, outbreaks that affect fewer persons are more likely to receive classifications of III or IV because of the limited sample size available for analysis. Changes in the 2003--2004 Surveillance SummaryNames, definitions, classifications, and other parameters in this Surveillance Summary have been modified and expanded to better reflect the changing epidemiology of WBDOs and to capture the wide scope of water-related disease. This section highlights these changes. Title The title of this Surveillance Summary has been changed. Previously titled Surveillance for Waterborne-Disease Outbreaks Associated with Recreational Water, the title of the report has been changed to Surveillance for Waterborne Disease and Outbreaks Associated with Recreational Water. This subtle difference ("Disease and Outbreaks") emphasizes the public health importance of certain waterborne contaminants (e.g., Naegleria, Vibrio, or chemicals) that frequently cause single cases of illness, can be strongly linked to recreational water exposure, and are reported to the WBDOSS, despite not being associated with multiple cases in a traditional "outbreak" setting. Etiologic Agents Etiologic agents are identified through clinical specimens or occasionally by water testing. In previous summaries, the term "acute gastrointestinal illness (AGI)" was used to indicate WBDOs of unidentified etiology associated with gastrointestinal symptoms. Because AGI refers to a type of illness and not to an etiologic agent, the term "unidentified" is now used to describe WBDOs with unknown etiology. A classification of "unidentified" might occur for various reasons, including a lack of clinical specimens, lack of appropriate testing, or inadequate laboratory capacity. If more than one agent is implicated, only those that appear in >5% of positive clinical specimens are included in the tables and calculations as etiologic agents. When each agent is of the same agent type (e.g., bacteria, chemicals/toxins, parasites, and viruses), the outbreak is analyzed within that agent type (e.g., an outbreak with both Cryptosporidium and Giardia would be analyzed as a parasitic outbreak). When agents represent more than one agent type, the outbreak is analyzed as a mixed agent outbreak. All outbreaks in which the etiologic agent is not known or confirmed are listed as unidentified, and they constitute a separate analysis category from those outbreaks with identified etiologic agents, even when other data (e.g., clinical findings) are suggestive of a particular pathogen or chemical/toxin. In previous Surveillance Summaries, outbreaks in which patients sought medical care for dermatologic symptoms consistent with Pseudomonas aeruginosa infection but in which Pseudomonas was not isolated from clinical specimens or water samples were still classified as Pseudomonas outbreaks. However, in this report, only those outbreaks in which clinical specimens or water samples test positive for Pseudomonas are classified as Pseudomonas outbreaks. Predominant Illness, Case Counts, and Deaths Whereas the illness associated with a WBDO generally includes only one category of symptoms (e.g., gastroenteritis), WBDOs do occur where the symptoms cluster into more than one category (e.g., gastroenteritis and dermatitis). Therefore, in this report, if any one illness category is reported by >50% of ill respondents, then multiple illnesses will be listed for that WBDO. These mixed-illness WBDOs constitute a separate analysis category from WBDOs involving a single illness. In addition, the number of deaths associated with each WBDO is now presented in this report. This change provides increased information on the severity of illness associated with each WBDO. Strength of Evidence Classification for Waterborne Disease and Outbreaks For the first time, the strength of evidence classification for WBDOs (Table 1) is used for nongastroenteritis outbreaks (e.g., dermatitis, PAM, and chemical/toxin poisonings). Classification of these WBDOs should provide a better understanding of the strength of each outbreak investigation. Vibrio Cases For the first time, single cases of recreational water-associated Vibrio illness were selected for inclusion in this Surveillance Summary by using an algorithm (Figure 1). The algorithm selected Vibrio cases for inclusion based on previous water exposure in the United States and the absence of seafood consumption or contact. All selected cases were verified by the state or local health departments. These infections frequently were associated with preexisting wounds but also were associated with other water-related exposure routes (e.g., wounds incurred while swimming or walking on the beach or unintentional inhalation of recreational water, resulting in a sinus infection). These cases are reported to the Cholera and Other Vibrio Illness Surveillance System on CDC form 52.79 (available at http://www.cdc.gov/foodborneoutbreaks/documents/cholera_vibrio_report.pdf). Staff operating the Cholera and Other Vibrio Illness Surveillance System collaborated with staff from the WBDOSS to gather all reported recreational water-associated Vibrio cases for inclusion in this report. These cases were analyzed separately from other recreational water illnesses to avoid substantially altering total WBDO numbers when compared with previous reports. Similarly, Vibrio cases are also discussed separately in this report. ResultsExcluding Vibrio cases, which are analyzed and discussed separately, a total of 62 outbreaks (28 in 2003 and 34 in 2004) associated with recreational water were reported to CDC (Tables 2--5). Of the 50 states and 10 territories, localities, and FAS participating in the WBDOSS, 27 (26 states and the territory of Guam) reported WBDOs (Figure 2). Descriptions of selected WBDOs have been presented (Appendix B, Selected Descriptions of Waterborne Disease and Outbreaks [WBDOs] Associated with Recreational Water). These 62 outbreaks resulted in 2,698 ill persons, including one death (attributable to PAM; Table 4). The median outbreak size was 14 persons (range: 1--617 persons). The seven largest outbreaks each had more than 100 ill persons and accounted for 60.3% (n = 1,628) of the total cases. Illinois reported the highest number of WBDOs (10), Ohio reported six WBDOs, and Georgia and Wisconsin both reported five WBDOs. During 2003--2004, treated water venues were associated with 43 (69.4%) of the recreational water outbreaks and 2,446 (90.7%) of the cases (Tables 2 and 3; Figure 3). Untreated venues were responsible for 19 (30.6%) of the WBDOs but only 252 (9.3%) of the cases (Tables 4 and 5). Similar proportions were identified by venue treatment type when gastroenteritis outbreaks were analyzed separately (Table 6). Of the 62 WBDOs, 30 (48.4%) were outbreaks of gastroenteritis, 13 (21.0%) were outbreaks of dermatitis, and seven (11.3%) were outbreaks of acute respiratory illness (ARI). The remaining WBDOs resulted in PAM (n = one), meningitis (n = one), leptospirosis (n = one), otitis externa (n = one), and mixed illnesses (n = eight) (Table 6, Figure 3). Gastroenteritis accounted for 1,945 (72.1%) of the cases of illness. The route of entry implicated for each WBDO was ingestion for 30 WBDOs (48.4%), contact for 15 (24.2%), inhalation for seven (11.3%), combined routes for eight (12.9%), other for one (1.6% [Naegleria]), and unknown for one outbreak (1.6%) (Figure 3). WBDOs occurred in every calendar month except October, but the summer months (June through August) accounted for 35 (56.5%) WBDOs and 1,888 (70.0%) cases (Figure 4). Gastroenteritis was particularly clustered during these months, in which 22 (73.3%) of 30 outbreaks and 1,631 (83.9%) of 1,945 cases (Figure 4) were reported. Treated venues were associated with WBDOs throughout the year, whereas untreated venue-associated WBDOs occurred almost exclusively from May through August (Tables 2--5). Increased reporting of WBDOs occurred during the summer, with a relative risk (RR) of 3.9 (95% confidence interval [CI] = 2.4--6.4). This risk increased for certain outbreak categories. Gastroenteritis outbreaks compared with other illnesses (RR = 8.2; 95% CI = 3.7--18.5) were especially frequent during the summer (Figure 4). Etiologic AgentsOf the 62 WBDOs associated with recreational water, the etiologic agent was confirmed in 44 (71.0%), suspected in 15 (24.2%) and unidentified in three (4.8%) (Table 7). Twenty (32.3%) outbreaks were confirmed as bacterial; 15 (24.2%), as parasitic; six (9.7%) as viral; and three (4.8%) as chemical- or toxin-mediated (Figure 3). Of the 43 outbreaks associated with treated water venues that had an identified etiologic agent, 14 (32.6%) involved bacteria; 12 (27.9%), parasites; four (9.3%), viruses; and one (2.3%), involved chemicals (Table 7). However, parasites were responsible for more than three times more cases than bacterial causes (1,414 versus 457). Of the 19 WBDOs associated with untreated water venues, six (31.6%) involved bacteria; three (15.8%) parasites; two (10.5%) viruses; and two (10.5%) toxins. Unlike treated water venues, bacteria were responsible for more than six times more cases in untreated water venues than parasites (96 versus 14). Parasites Of the 30 outbreaks of gastroenteritis, 14 (46.7%) were parasitic in origin, including 11 (78.6%) caused by Cryptosporidium, two (14.3%) caused by Giardia intestinalis, and one (7.1%) caused by both Cryptosporidium and Giardia (Tables 2--6; Figure 5). Of the 12 gastroenteritis outbreaks associated with untreated water venues, only two (16.6%) were caused by parasites. A single Cryptosporidium outbreak and a single Giardia outbreak each occurred in untreated lake water, causing four and nine cases of illness, respectively. In contrast, parasites were the most common causes of gastroenteritis outbreaks associated with treated water venues; Cryptosporidium was the most common parasitic agent, causing 10 (55.6%) of the 18 outbreaks. A total of 12 parasitic gastroenteritis outbreaks occurred in treated water venues that caused illness in 1,414 persons. Four of these outbreaks each caused over 100 (range: 149--617 persons) cases of illness. In June 2003, an outbreak of G. intestinalis started at a Massachusetts membership club pool and resulted in 149 cases, including cases of secondary person-to-person transmission. In July 2003, a C. hominis outbreak spread in multiple Kansas pools and day care centers and resulted in 617 cases; this outbreak was the largest recreational water outbreak during 2003--2004. In July 2004, an outbreak of Cryptosporidium in a community pool in Ohio caused gastroenteritis in 160 persons from three counties. In August 2004, employees ill with gastroenteritis at a California water park continued working and swimming in the pools, resulting in a Cryptosporidium outbreak involving 336 persons. Of the 15 WBDOs of all illness types confirmed to be of parasitic origin, only one (6.7%) did not involve gastroenteritis; a single fatal case of PAM caused by Naegleria fowleri occurred in July 2003 at a lake in North Carolina. This case was the only death reported among the 62 WBDOs during this reporting cycle (excluding Vibrio cases). Bacteria Six reported gastroenteritis outbreaks of confirmed bacterial origin were reported (Figure 5), one of which was at a treated water venue. This outbreak of Shigella sonnei occurred in an interactive fountain in Oregon in July 2003, resulting in 56 cases. Inadequate disinfection, poor monitoring of water chemistry, and heavy use of the fountain by young diaper-aged children were all cited as factors contributing to the outbreak. The other five bacterial outbreaks of gastroenteritis were associated with untreated bodies of water, including two additional outbreaks of Shigella, two outbreaks of Plesiomonas shigelloides, and one outbreak that involved both Shigella and Plesiomonas associated with the use of a lake in Maryland, resulting in illness in 65 persons. Fecal accidents and sewage contamination were implicated in this outbreak. The other four outbreaks were substantially smaller; illness occurred in 13 or fewer persons in each outbreak. Nine of the bacterial outbreaks resulted in cases of dermatitis; for eight of these outbreaks, Pseudomonas aeruginosa was the confirmed etiologic agent. Three of the eight Pseudomonas outbreaks were associated with mixed illnesses. All eight Pseudomonas outbreaks occurred at treated water venues that involved heated spa water (some of these outbreaks also involved pools), and illness occurred in 274 persons. One outbreak in Ohio in July 2004 involving a spa and pool accounted for 119 of these cases, which is the largest bacterial outbreak summarized in this report. Potential exposure also occurred in this outbreak when the hotel spa water flowed directly into the swimming pool. The one bacterial dermatitis outbreak that did not involve Pseudomonas occurred in August 2003. Multiple members of a Connecticut college football team were diagnosed with methicillin-resistant Staphylococcus aureus (MRSA) skin infections. A spa at the team's athletic facility, which was disinfected with an unapproved disinfectant (i.e., povidone), was implicated in the outbreak. Four outbreaks caused by Legionella pneumophila were associated with treated recreational water venues (i.e., spas) during 2003--2004. Three of these outbreaks each had fewer than five cases of Legionnaires' disease. The fourth outbreak, which occurred at a hotel in Oklahoma during a weeklong basketball tournament in March 2004, included six cases of Legionnaires' disease and 101 cases of PF. The bather load (i.e., maximum occupancy) of the hotel spa was exceeded, and the bromine concentrations in the spa were not adequately monitored. An April 2004 outbreak of leptospirosis in Guam involved three U.S. military personnel who swam in a remote set of waterfalls. This was the only outbreak of leptospirosis reported and the only outbreak reported from outside the 50 states. Viruses Six outbreaks of confirmed viral origin occurred, five of which caused gastroenteritis. In all five of these gastroenteritis outbreaks, norovirus was identified as the etiologic agent; two occurred at lake swimming beaches, and three occurred in treated water settings. These five norovirus outbreaks resulted in 300 cases of gastroenteritis. Three other outbreaks were suspected to have been caused by norovirus contamination. One outbreak (Idaho, March 2004) occurred during a swimming competition at a community pool and resulted in 140 cases. One outbreak (Florida, May 2004, norovirus etiology) involved an elementary school that used an outdoor hose to supply waterslides during outdoor play time. Children were infected after one ill child with diarrhea used one of the slides; secondary transmission to household contacts also occurred, resulting in 42 cases. In July 2003, a viral outbreak of meningitis occurred in a pool at a Connecticut campground. Echovirus 9, an enterovirus, was isolated from patient cerebrospinal fluid samples. Although aseptic meningitis occurred in 12 of 36 persons, a wide range of other symptoms were reported by the other 24 ill persons, including headache and rash. Chemicals/Toxins During 2003--2004, three outbreaks involving chemicals or toxins resulted in 25 ill persons. One outbreak occurred in a treated water venue. In March 2003, muriatic (i.e., hydrochloric) acid, used for pH control in recreational water, spilled on the floor at an indoor pool in New York and resulted in exposure to toxic fumes, which led to respiratory distress in three persons who sought emergency department medical care. During 2004, two toxin-associated outbreaks occurred in untreated water venues in Nebraska. These outbreaks were attributed to elevated levels of microcystin toxin (17) from blue-green algae (i.e., cyanobacteria) in lakes, causing 22 cases of illness. The predominant illnesses in both outbreaks involved dermatitis and gastroenteritis. Patients who sought medical care had a combination of rashes, diarrhea, cramps, nausea, vomiting, and fevers. Unidentified Etiologic Agents Eighteen outbreaks occurred in which no etiologic agent was confirmed; however, in 15 of these outbreaks, investigation reports described a suspected agent, based on symptoms, setting, and circumstances (Table 7). Of these 18 outbreaks, seven reported skin infections, five reported gastroenteritis, three reported mixed-eye and ARI, two reported ARI, and one reported ear infections. Eight of these 15 outbreaks were suspected to be related to chemical exposure. For one of these outbreaks (Georgia, January 2004), psychogenic factors also were suspected to play a role in the 17 cases of dermatitis because certain rashes resolved before first responders arrived to investigate. Another outbreak of gastroenteritis was suspected to be a result of the application of a pool algaecide before swimmers entered the pool. The six remaining outbreaks all were suspected to involve exposure to excess chloramines (i.e., disinfection by-products of chlorination) (18--20) in the indoor pools and surrounding areas (i.e., indoor pool air), which resulted in ARI, eye irritation, and gastroenteritis. P. aeruginosa was the suspected pathogen in two dermatitis outbreaks in treated water venues. Norovirus was the suspected pathogen in three gastroenteritis outbreaks at lakes, based on epidemiologic and clinical evidence. Two outbreaks were suspected to be the result of contact with avian schistosomes, causing cercarial dermatitis. Information regarding the remaining three outbreaks of unidentified etiology was not sufficient to suggest an etiologic agent. Skin infections were reported as the predominant illness in two of these outbreaks, and ear infections were reported for the third one. Two outbreaks of skin infections were associated with spas. One resulted in 64 ill persons, but water sampling could not be conducted because the spa had been drained for routine maintenance before the investigation (South Carolina, November 2003). The third outbreak resulted in ear infections in nine children (Georgia, August 2004) who had been swimming and submerging their heads in a lake. Vibrio Cases Associated with Recreational Water During 2003--2004, a total of 142 Vibrio cases associated with recreational water were reported from 16 states. Recreational water-associated Vibrio cases were defined as those with recreational water exposure in the United States before infection and with no evidence that contact with seafood or marine life might have caused infection (Figure 1). Among patients for whom information was available, 70 (49.3%) of 142 were hospitalized, and nine (6.3%) of 142 died (Table 8). The most frequently isolated Vibrio species was V. vulnificus, which was isolated from 47 (33.1%) persons; 41 (87.2%) were hospitalized, and six (12.8%) died. V. alginolyticus was isolated from 43 (30.2%) persons; eight (18.6%) were hospitalized, and one (2.3%) died. V. parahaemolyticus was isolated from 34 (23.9%) persons; 15 (44.1%) were hospitalized, and none died. Other Vibrio species (including noncholerigenic V. cholerae, V. damsela, V. fluvialis, nonspeciated Vibrio, and mixed Vibrio species) were identified in 18 (12.7%) persons; six (33.3%) were hospitalized, and two (11.1%) died. Six patients were reported to have had an amputation; five were infected with V. vulnificus; and one with V. parahaemolyticus. Other bacterial species also were identified with Vibrio; 25 (25.3%) of 99 Vibrio isolates for which information was available yielded other bacterial species. These other species included E. coli, Pseudomonas species, Staphylococcus marcescens, S. aureus, and Streptococcus. Of the 149 Vibrio isolates taken from 142 patients, 85 (57%) were from wounds, 31 (20.8%) from blood, 27 (18.1 %) from ears, and six (4%) from other sites (i.e., chest abscess, eye, incision, sinus, sputum, stool, and urine). Geographic location. Nearly all Vibrio patients reported that they were exposed to recreational water in a coastal state (Figure 6). The most frequently reported location was the Gulf Coast (62.7%); Pacific Coast states (19.7%); Atlantic Coast states, excluding Florida (16.9%); and inland states (0.7%) (Table 9). Florida, Hawaii, and Texas reported the highest number of cases, 51, 23, and 28 cases, respectively (Figure 6; Table 9). Seasonality. In the temporal distribution of illness in patients from whom Vibrio species were isolated, a clear seasonal peak occurred during the summer (Figure 7). The greatest frequency of Vibrio cases occurred during July and August for all species. Exposures. Activities associated with Vibrio cases included swimming, diving, or wading in water (66.9%); walking or falling on the shore or rocks (32.3%); and boating, skiing, or surfing (21.8%). The majority of patients reported being exposed in the ocean (100 [70.4%]); 12 (8.5%) were exposed in a river, stream, or creek; seven (4.9%) were exposed in a lake or bay; eight (5.6%) were exposed to another water source; and 15 (10.6%) exposed a wound to an unknown water source. Symptoms. Symptoms associated with Vibrio cases were cellulitis (54.9%), fever (41.5%), muscle pain (24.6%), ear infection (19.0%), nausea (18.3%), shock (12.7%), and bullae (12.0%) (Figure 8). V. vulnificus accounted for the majority of skin infections, including cellulitis, bullae, and other skin infections (56 [51.9%] of 108). V. vulnificus also accounted for the majority of severe illnesses, including those with fever (79.5%), bacteremia (80.6%), and shock (66.7%). V. alginolyticus accounted for the majority of ear infections (17 [63.0%] of 27). Other symptoms and infections were reported in low frequencies (e.g., bladder infections, hematuria, eye infections, respiratory symptoms, sinus infections, diarrhea, and vomiting). Previously Unreported OutbreakOne previously unreported recreational water outbreak from 2002 was received. The outbreak is summarized but not analyzed in this Surveillance Summary. The outbreak occurred in Florida in December 2002 and involved two laboratory-confirmed cases of Legionnaires' disease (i.e., Legionella pneumophila serogroup 1) linked to a hotel spa. Both persons were hospitalized and recovered. No Legionellae were recovered from the spa, but epidemiologic evidence (Class III) implicated the spa as the probable source for this cluster of cases. Bromine tablets were used to disinfect the spa, but the tablets did not dissolve properly, leading to low bromine concentrations in the water and conditions favorable for the growth of Legionella. DiscussionTrends in Reporting OutbreaksA total of 62 recreational water-associated WBDOs were reported to CDC during 2003--2004. This number is a slight decrease from the previous 2001--2002 Surveillance Summary in which a record number (65) of WBDOs were reported. Both the number of reported recreational water-associated WBDOs (Pearson's correlation = 0.59; p<0.01) and outbreaks of gastroenteritis (Pearson's correlation = 0.86; p<0.01) have increased significantly since 1978 when CDC first began receiving these reports (Figure 9). These increases are likely a result of a combination of factors such as the emergence of pathogens (e.g., Cryptosporidium), increased participation in aquatic activities, and increases in the number of aquatic venues. Increased recognition, investigation, and reporting of recreational water-associated outbreaks also might be contributing factors. The number of reported WBDOs also differs substantially based on geographic location (Figure 2). This variation might be a result of several factors, including public awareness of the outbreak, availability of laboratory testing, requirements for reporting diseases, and resources available to local and state health departments for surveillance and investigation of probable outbreaks. Differences in the capacity of local and state public health agencies and laboratories to detect WBDOs probably result in reporting and surveillance bias. Therefore, the states with the majority of outbreaks reported for this period might not be the states in which the majority of outbreaks actually occurred. An increase or decrease in the number of WBDOs reported might reflect either an actual change in the incidence of outbreaks or a change in the sensitivity of surveillance practices. Multiple other factors also might influence which WBDOs are reported. Larger outbreaks are more likely to be identified by public health authorities and to receive more rigorous investigations. Etiologic agents with shorter incubation periods might be more easily linked to water exposures, facilitating the recognition of outbreaks. In contrast, private residential pools and spas might experience problems that go undetected because they are not regulated or inspected by public health agencies. In addition, outbreaks of gastroenteritis at large venues that draw from a wide geographic range (e.g., the Great Lakes and ocean beaches) might be difficult to detect because potentially infected persons disperse widely from the site of exposure and, therefore, might be less likely to be identified as part of an outbreak. Such an effect is supported by data from EPA's NEEAR Water Study (16). This prospective study of large beaches on the Great Lakes has indicated that elevated rates of gastroenteritis have occurred in swimmers compared with nonswimmers on all four beaches studied, although outbreaks associated with the use of the beaches were not reported during this period. Consistent with this finding, WBDOs reported in Surveillance Summaries have not been ocean beach-associated outbreaks of gastroenteritis, and only one Great Lakes beach-associated outbreak of gastroenteritis has been reported since 1978 (2). Multiple other prospective studies of gastroenteritis associated with beach swimming have also indicated elevated rates of illness associated with swimming (21). This endemic recreational water-associated illness is not captured by the WBDOSS, supporting the need for more studies to be conducted to determine the magnitude of risk of illness for routine, nonoutbreak-associated exposures at recreational water venues. WBDOs associated with recreational water use occur year-round, but the number of reported WBDOs and cases are highest during the annual summer swim season (Figure 4). For public health professionals, these trends can help determine the allocation of resources so that health education messages are targeted to populations during times of the year when the highest risk for preventable illness occurs. Swimming PoolsInfectious Gastroenteritis During 2003--2004, Cryptosporidium caused the largest number of recreational water-associated outbreaks (n = 11). These outbreaks accounted for the largest number of ill persons included in this report (n = 1206); 99.7% of these cases were associated with treated water venues. During 1995--2004, Cryptosporidium was implicated in 39.0% of the recreational water-associated outbreaks of gastroenteritis and, although Cryptosporidium rarely was attributed to outbreaks in lakes and rivers (10% of outbreaks), it caused 61.8% of outbreaks associated with treated venues (Figure 10). This observation for treated venues is consistent with the finding that Cryptosporidium requires extended contact time with chlorine for inactivation; oocysts can survive for days in the chlorine levels that typically are recommended for swimming pools (1--3 ppm free chlorine; 22). The continued reporting of cryptosporidiosis associated with the use of treated water venues underscores the importance of other prevention measures that reach beyond traditional pool chlorination, which is currently the primary barrier to infectious disease transmission. Cryptosporidiosis has stimulated the need for new technology to keep swimming venues safe (e.g., ultraviolet light irradiation, ozonation, chlorine dioxide use, or improved filtration). However, cryptosporidiosis outbreaks also highlight the need for improved operator training and continued education of the general public concerning appropriate healthy swimming practices to reduce the risk of future outbreaks. Because Cryptosporidium is resistant to the chlorine levels used in pools, outbreaks can occur, even in facilities that are well-maintained. Therefore, a rapid public health response and increased community involvement is needed to prevent the expansion of these outbreaks (23). The Cryptosporidium outbreak (Ohio, July 2004) that occurred in a community swimming pool demonstrates that a rapid communitywide public health response during the early stages of an outbreak can help control the potential spread of illness into the community. In Ohio, detection and investigation started during the second week after exposure. The response included mitigating actions (e.g., hyperchlorination of all pools and providing instructions regarding proper water hygiene to pool staff and users, day care centers, restaurants, and other potentially affected facilities). In addition, the investigation indicated that no transmission had apparently occurred outside of the single community pool. In contrast, the outbreak in Kansas (July 2003) was not detected for multiple weeks. As a result, a full communitywide outbreak occurred when ill pool patrons and daycare center attendees continued their normal activities (despite their illness) exposing large numbers of persons to Cryptosporidium. Approximately 60% of all cases of illness reported to this surveillance system during 2003--2004 were associated with infectious gastroenteritis outbreaks in treated pools. Several of these outbreaks (e.g., giardiasis, norovirus, and echovirus) could have been prevented or reduced in scale by using proper pool disinfectant procedures and by following existing operation, maintenance, and communication protocols because of the pathogens' chlorine sensitivities. The norovirus outbreak (Vermont, February 2004) demonstrated that when pool staff do not follow these protocols, outbreaks might occur. Despite complaints from patrons concerning water quality, on-duty staff failed to alert off-duty pool operation personnel. As a result, a malfunctioning chlorinator system was not detected for several days; norovirus transmission occurred for multiple days (which probably would have been hours or less with proper chlorination) before the breakdown was discovered and corrected (24). This outbreak emphasizes the need for both effective communication channels at aquatic facilities and trained personnel on site or accessible on weekends when pool use is highest. Swimming behavior is also a critical component of pool operation. Because swimming is essentially communal bathing, when persons who are ill with infectious diarrhea continue to swim, a public health challenge is created that requires a focused public education effort. In addition, improved hygiene is essential to ensure the cleanliness of swimmers entering pools. Functioning and adequate hygiene facilities (i.e., toilets, diaper-changing areas, and showers) in adequate numbers should be located near pools and should provide hot water and handwashing access. Swimmers should be encouraged to shower thoroughly (i.e., washing the perianal surface in particular) before entering the pool. Diaper-changing facilities, with hand-washing stations, should be readily accessible to prevent diaper-changing at the poolside. These outbreaks demonstrate how pools can serve as ideal amplification venues for fecal-oral transmission of pathogens. As a result, facilities should be diligent about making patrons aware of these public health concerns and about making clear that "no diarrhea" policies apply to all pools. This policy is especially needed for young children visiting pools, particularly large groups (e.g., day care centers), which already have diarrhea exclusion policies but might not always enforce them (Kansas, July 2003). Diarrhea exclusion policies should apply to both pool employees when swimming and food workers when preparing food. For the waterpark-associated outbreak in California (August 2004), documentation revealed that employees were ill with diarrhea before the main outbreak, which involved patrons, and that employees admitted to swimming while symptomatic. All aquatic facilities need to establish standardized policies for keeping staff who are ill with diarrhea out of pools and should subsequently implement and enforce these policies. Meningitis Although gastroenteritis is the most common illness spread via pool outbreaks, it is not the only disease that can be contracted in this manner. In one outbreak, the transmission of an agent causing viral meningitis via a swimming pool at a recreational vehicle campground (Connecticut, July 2003) was reported. The implicated enterovirus, Echovirus 9, was the predominant enterovirus serotype circulating through the eastern United States during 2003 and is susceptible to chlorine if proper chlorine residuals are maintained (25). Properly monitored and maintained chlorination levels and pH control in pools should prevent this type of WBDO. Chemical Toxicity During 2003--2004, pool chemicals or disinfection by-products were confirmed (n = one) or suspected (n = eight) in nine pool-associated outbreaks. Chemicals are added to pool water to protect against microbial growth and improve the water quality and efficacy of the disinfection process (e.g., pH control). However, these same chemicals can become sources of illness if they are not properly handled or if water quality and ventilation are poor. One outbreak in New York (March 2003) involved an overflow and spill of muriatic (i.e., hydrochloric) acid, which is used for pH control of pool water. As a result, three persons developed ARI from exposure to fumes. Another outbreak (Illinois, July 2004) was suspected to be caused by ingestion of algaecide that was added to the pool before a swim meet, which resulted in nine persons becoming ill with gastroenteritis. These outbreaks underscore the need for safe chemical training (i.e., adding disinfectant, controlling pH levels, and using pool additives appropriately), handling, and safety practices at all aquatic facilities to protect the health of patrons and staff. These policies should include proper handling of chemicals in the pump room and application procedures for adding pool chemicals directly to the pool. Six outbreaks of acute respiratory symptoms, eye irritation, and gastroenteritis were suspected to be a result of an accumulation of chloramines in the air and water of indoor pools. Chloramines are disinfection by-products that result from chlorine oxidation of nitrogenous waste compounds, commonly shed into pools by swimmers (e.g., perspiration, saliva, urine, and body oils) (18). These chemicals are produced in the water and volatilize in the air. In indoor pool settings, chloramines can also accumulate in the enclosed spaces if ventilation is inadequate (19). The resulting high levels of chloramines can cause respiratory tract and mucous membrane irritation (20); these high levels also are potentially linked to asthma in indoor pool settings (26). Because of the shortage of laboratories that perform analyses for airborne chloramines and because of rapid shifts in indoor air quality over days, the investigators' ability to respond to reports of airborne chloramines and to quantitatively identify these chemicals is difficult. Investigators should always document the easily measured total chlorine concentration (i.e., free plus combined chlorine) and free chlorine levels of the pool water to obtain some indication of pool water quality and the potential for the presence of disinfection by-products, especially chloramines, which might be present in the water and air. Multiple steps can be taken to address indoor pool problems, including swimmer behavior modification. Encouraging showering before entering any pool or spa and facilitating frequent bathroom breaks for swimmers, particularly young children (i.e., by instituting adult-only swim times and short closures for water-quality testing), might reduce the amount of 1) urine and other nitrogenous waste contaminating the water and 2) accumulation of chloramines. To encourage swimmers to refrain from urinating in public pools, they should be educated that stinging eyes from pool chemicals are actually caused by human waste (i.e., urine and sweat) in the pool water. Improved indoor pool ventilation is vital to increase air-turnover and to remove concentrated chloramines; however, new studies have suggested that installation of ultraviolet light treatment devices in pool water recirculation systems can reduce pool chloramine levels and inactivate chlorine-resistant pathogens (e.g., Cryptosporidium) (27,28). Surveillance for recreational water-associated outbreaks of acute chemical poisonings is likely to have multiple barriers; therefore, the number of reported chemical/toxin WBDOs probably underestimates the true magnitude of the problem. Symptoms associated with chemical poisonings in recreational water settings might be substantially different from those associated with more familiar infectious microbes, which might lead to decreased chemical-related WBDO identification. By contrast, chemicals/toxins and infectious agents might cause similar symptoms (e.g., gastrointestinal illness), and investigators might fail to identify the etiologic agent because they do not suspect a chemical etiology. Multiple health departments use infectious disease epidemiologists for WBDO surveillance and investigation. However, chemical-related WBDOs and recreational WBDOs, in general, might be investigated by staff from different sections of the health department or by staff from different agencies. Because of the acute nature of certain chemical-related WBDOs, first responders will likely be called to the scene, and persons from these agencies might be less likely to report back through the traditional chain of health department infectious disease epidemiologists who report to the WBDOSS. Therefore, building strong and effective intra- and interagency communication networks between health departments and other groups (e.g., first responders and pool operators) to reduce the underreporting of recreational WBDOs is critical. SpasSpas are susceptible to contamination from persons infected with the same pathogens that cause gastroenteritis in swimming and wading pools. However, the increased temperature of the water also makes these venues susceptible to contamination with and amplification of thermophilic pathogens (e.g., Pseudomonas and Legionella) that naturally occur in the environment (i.e., contamination does not necessarily occur via ill swimmers). Skin Infections Spa-associated outbreaks are commonly associated with dermatitis and folliculitis; P. aeruginosa is the most commonly reported agent implicated in these settings (29). In this report, eight confirmed Pseudomonas WBDOs and two suspected Pseudomonas WBDOs were documented; five of these outbreaks involved spas, one involved a pool, and four involved both spas and pools. Because of the frequent use of both spas and pools at the facilities, determining whether the spa, pool, or both are implicated in transmission of illness is epidemiologically difficult, although amplification of Pseudomonas is more likely to occur in the higher temperatures of spas. One outbreak report (Ohio, July 2004) concluded that Pseudomonas growing in a spa was transferred to a pool through combined water circulation and that infection occurred in both settings. Spas are a challenge to maintain and operate because they typically have reduced bather capacity compared with swimming pools, so they can more easily be overloaded and rapidly lose disinfectant concentrations when bather loads exceed recommended numbers of persons. In addition, depletion of disinfectant levels is increased at higher temperatures. Large gatherings at hotels and motels with spas (e.g., cheerleading competitions [North Carolina, March 2004], dance competitions [Ohio, July 2004], and school class outings [Michigan, February 2003]) can rapidly overload the disinfection capacity and lead to bacterial amplification. In addition to overloading the spas and depleting the disinfectant, these groups frequently arrive on weekends when hotel staff trained in spa maintenance are off duty. Hotels and motels should consider that employees with appropriate pool and spa operation training are needed on weekends, when usage is typically highest. Enhanced monitoring and maintenance should be implemented when a large group or event at a hotel is scheduled. Multiple aquatic facilities have transitioned to employing remote monitoring services to check pool chemistry (e.g., chlorine and pH) on a regular basis and to alert the facility of any problems that arise. Breakdowns in communication between these remote monitoring services and the aquatic facility seem to facilitate problems that occur for long periods, without correction, which was documented in a large outbreak of Pseudomonas dermatitis in Illinois (January 2003) and several previous outbreaks (30). Facilities should not rely on off-site monitoring companies as the sole overseers of their aquatic facilities. Although remote monitoring can be beneficial in detecting water-quality problems, the service should not take the place of routine water-quality checks, which are required in the majority of pool codes. To prevent adverse events, having 1) clear communication plans for relaying warnings concerning problems, 2) prompt alerts so corrections can be made, and 3) diligent staff who immediately respond to alerts are essential. To prevent spa-associated outbreaks, understanding the risk factors and steps that can be taken is necessary to limit transmission of the bacteria. Proper chlorination or bromination is effective in killing Pseudomonas and other skin-infecting bacteria. However, sufficient chlorine and bromine levels must be maintained consistently along with adequate pH control to limit bacterial amplification. Poor maintenance of spas has been documented (31). Cycling between high and low disinfectant levels allows biofilms to proliferate on spa surfaces, creating an environment where Pseudomonas and other bacteria are protected from disinfection (32). A review of 18 Pseudomonas outbreaks has demonstrated that all spa-associated outbreaks had inadequate disinfection (33). The majority of Pseudomonas outbreaks can be prevented by properly maintaining spas and by ensuring that disinfectant levels remain >1 ppm and pH levels remain in a range of 7.2--7.8. In addition, elimination of potential sources of Pseudomonas (e.g., soil from potted plants in close proximity to the water) (Illinois, January 2003) is advisable. Pseudomonas is not the only bacterium that can cause spa-related skin infections. MRSA was associated with an outbreak involving an athletic spa in Connecticut (August 2003; 34). MRSA infections can have substantial consequences, as in this outbreak in which otherwise healthy young athletes were hospitalized. Factors contributing to this outbreak included the presence of skin abrasions on the athletes from "turf burns" and body shaving, and the communal use of an athletic spa that employed limited and unproven disinfection methods. Appropriate spa operation, maintenance, and cleaning should prevent outbreaks of this emerging infectious disease. Legionellosis Legionellae, which cause both Legionnaires' disease and PF, are ubiquitous in freshwater environments (35). However, certain environmental conditions in spas (e.g., high temperatures and water aerosolization) promote the amplification and transmission of the bacteria. Similar to outbreaks of Pseudomonas dermatitis associated with spas, transmission of Legionella is more likely to occur in the absence of adequate levels of disinfectant, underscoring the importance of maintaining disinfectant levels and pH control. When lapses in preventive measures occur and Legionella outbreaks occur, morbidity can be reduced by rapid recognition of the outbreak, identification of its source, and immediate implementation of remediation. These methods include cleaning and disinfecting the spa to eliminate Legionella colonization and performing follow-up cultures of Legionella to ensure that regrowth does not occur (36). Of the four Legionella WBDOs associated with recreational water during 2003--2004 (as well as one from 2002 which was previously unreported), all except one were associated with hotel spas. These travel-associated WBDOs highlight the importance of timely reporting of individual cases of legionellosis, which was recently recommended in a 2005 CSTE position statement (http://www.cste.org/PS/2005pdf/final2005/05-ID-01final.pdf). Interactive Fountains/Wet Decks and WaterslidesInfectious Gastroenteritis Certain treated water venues (e.g., interactive fountains, which are also called wet decks) might be overlooked as potential sites for disease transmission or pool regulation because they do not have the standing water found in traditional swimming pools. Outbreaks in this report continue to demonstrate the possibility of infection occurring in these settings. The use of interactive fountains has previously been associated with outbreaks of gastroenteritis (37). In two WBDOs in this report, contaminated interactive fountains are implicated; one involved a S. sonnei--contaminated fountain (Oregon, July 2003), and the other involved a Cryptosporidium-contaminated (Illinois, July 2004) fountain and swimming pool. In certain states, interactive fountains are not regulated as other recreational water venues, and fountain designs that include recirculation of the bathing water make these venues vulnerable to contamination. New designs that improve water treatment for these interactive fountains are needed so that visitors can enjoy them without risk from waterborne diseases. The traditional use of tap water to fill or operate temporary aquatic venues (e.g., wading pools and waterslides) used by young children also needs to be reconsidered, particularly in institutional settings (e.g., day care centers and schools). If the water is not treated with adequate levels of disinfectant, residual disinfectant in the water is rapidly depleted; users are then at higher risk of exposure to infectious microbes in the untreated water. Special consideration needs to be given to kiddie pools, some of which have had unfavorable water-quality test results and associations with previous outbreaks (38). In one Cryptosporidium outbreak (Iowa, June 2003), a kiddie pool at a day care facility was filled with potable municipal water that had not received additional treatment, expediting infection of children and eventual expansion into a communitywide outbreak. In Kansas (July 2003), the communitywide outbreak also involved use of kiddie wading pools and in-ground pools at local day care centers. Portable waterslides in which municipal water is used also might be overlooked as sources of disease transmission because they can be set up, used, and taken down in a matter of hours. The outbreak in Florida associated with a waterslide (May 2004) demonstrated that the use of these slides by a person infected with a fecal-oral transmissible microbe (in this case, norovirus) contaminated the waterslide, so it became an ideal venue for spreading disease. As with pools, spas, and fountains, appropriate treatment of recreational water venues and exclusion of persons with diarrhea is needed to prevent disease transmission. Furthermore, the use of temporary pools filled with municipal water that do not include routine disinfection and filtration should be considered carefully by the public and, based on documented outbreaks, should be eliminated from institutional settings (e.g., day care centers and schools). Lakes and RiversInfectious Gastroenteritis Since the WBDOSS began collecting data on recreational water outbreaks, reports have implicated both treated and untreated venues. Since 1998, the numbers of reported outbreaks from treated water venues have surpassed those from untreated venues (Figure 11). For 2003--2004, a total of 12 outbreaks of gastroenteritis associated with untreated freshwater venues were reported; 11 of these outbreaks involved lakes, and one involved a reservoir. Freshwater outbreaks were more likely to be of a bacterial or viral origin than treated water outbreaks (Figure 5). As with treated venues, human behavior plays a role in the spread of pathogens in untreated bodies of water. For example, in an outbreak in Maryland (July 2003), 5--10 diapers were reportedly retrieved from the lake each week. Modification of swimmer behavior might be a more critical factor because these natural water venues do not have the benefit of disinfection and filtration barriers. Recommendations for swimmer hygiene are the same for lakes, as previously discussed regarding treated pools. In addition, beach managers and swimmers should be informed that shallow swimming areas with poor water circulation, although desirable to many swimmers, might pose a higher risk if a swimmer contaminates the water. Use of methods to improve circulation of water through these beach areas should be explored for the potential to reduce the risk for waterborne disease transmission. Additional reduction of risk might be accomplished by avoiding swimming immediately after a heavy rainfall when the water is at higher risk for transient contamination, and by avoiding swimming near storm drains or pipes that might release sewage into bodies of water. The use of water-quality monitoring (e.g., fecal indicator testing) by beach managers might also reduce risk (15), particularly when more rapid testing methods are implemented by EPA (16). Primary Amebic Meningoencephalitis Whereas infection with Naegleria fowleri, the cause of primary meningoencephalitis (PAM), is a rare occurrence in the United States (39), this disease has public health importance because of its high fatality rate. This free-living ameba proliferates in warm freshwater and hot springs. PAM is caused when the ameba coincidentally enters the nasal passages, travels to the olfactory lobe of the brain, and infects brain tissue. Only one fatality (North Carolina, July 2003) was reported for this 2003--2004 surveillance cycle (excluding Vibrio illnesses). During the summer, a young child was exposed to infection through warm lake water, similar to cases of PAM during previous years. PAM is difficult to predict, and prevention strategies might not prevent these tragic events. However, swimmers potentially can reduce their risk of PAM by wearing nose plugs, holding their nose while diving or jumping into the water, refraining from digging in sediment, and avoiding swimming in shallow waters during the warmest times of the year. Additional resources are needed to develop more evidence-based prevention measures. Leptospirosis Leptospirosis infection occurs worldwide, except in polar regions, and particularly in tropical and semitropical areas of the world, including several of the Pacific islands that report to this surveillance system (40). Leptospira can be found in the urine of infected wild and domesticated animals. Human infection can occur when contaminated water is ingested, aerosolized droplets are inhaled, or water enters the body through skin abrasions. One outbreak of leptospirosis was reported for 2003--2004 and involved three cases, which resulted from exposure to a river and waterfalls in Guam (April 2004). This outbreak occurred among U.S. military personnel in a remote area, and water buffalo moving through that region were suspected to have been the possible sources of contamination. Blue-Green Algae Toxicity Toxin or chemical-associated outbreaks can occur by natural mechanisms. Blue-green algae that bloom in freshwater lakes have been identified as sources of outbreaks of human waterborne diseases in multiple countries (41). The toxins involved include anatoxin (i.e., a potent neurotoxin) and microcystins (i.e., potent liver toxins), and poisonings can cause various symptoms. These symptoms were observed in two outbreaks (Nebraska, 2004) in which 22 persons became ill with ARI and dermatologic symptoms. The actual number of persons who become ill after exposure to blue-green algal toxins in drinking and recreational waters is not known; substantial research is needed to identify the actual extent of this public health threat. Toxin levels can be measured in samples collected from lakes where blooms occur. Currently no regulations exist that establish acceptable toxin levels in drinking or recreational water. Cercarial Dermatitis During the 2003--2004 surveillance period, two WBDOs of suspected cercarial dermatitis caused by avian schistosomes were reported (California, June 2003; Ohio, June 2003). Although the diagnosis was not confirmed, this self-limited disease is known to occur in lakes across America where the intermediate host snail species are found and a population of suitable bird hosts are present (42). Cases of cercarial dermatitis might be reduced by posting warning signs at lakes known to be infested, avoiding shallow swimming areas where infected snails reside, instituting a snail-control program, and by not attracting birds to swimming areas (e.g., by feeding them). Marine WaterVibrio Illness A limited number of outbreaks at marine venues have been reported to the WBDOSS. Outbreaks in these settings can be difficult to detect because persons affected frequently travel from distant locations to visit these venues and might disperse before a health problem is recognized. However, single cases of Vibrio infections from recreational water exposure are captured through the Cholera and Other Vibrio Illness Surveillance System (http://www.cdc.gov/foodborneoutbreaks/vibrio_sum/cstevibrio2004.pdf) and represent an essential aspect of waterborne morbidity and mortality in the United States. As a result, recreational water-associated Vibrio illnesses will now be included in the WBDOSS to report the scope of waterborne disease in the United States in a more comprehensive manner. During 2003--2004, the most commonly reported species were V. vulnificus, V. alginolyticus, and V. parahaemolyticus. Of these species, V. vulnificus illnesses had the highest hospitalization rate (87.2%) and mortality rate (i.e., 12.8% of infected patients with recreational water exposure). The predominant syndrome associated with Vibrio illness caused by recreational water was wound infection. Vibrio wound infections were characterized by cellulitis, muscle pain, and especially with V. vulnificus, bullae, and septicemia. Vibrio illness caused by recreational water exposures occurs in all regions of the United States but most frequently occur along the Gulf Coast. However, the majority of V. alginolyticus cases occur in the Pacific coast states, where the most common exposures occur through surfing and swimming. Improved surveillance and analysis is needed to 1) assess the actual magnitude of Vibrio illness and other WBDOs at marine water venues, 2) better characterize the risk, and 3) educate the public concerning appropriate prevention measures (e.g., not swimming in warm water when a person has an open wound). PreventionPrevention of recreational water illnesses is likely to be accomplished only through a concerted team effort by public health professionals and swimming venue operators to educate all persons involved in recreational water activities, including the general public, concerning appropriate prevention measures. Operators at treated water venues are equipped with various methods that should be employed to prevent outbreaks. The traditional reliance on two water-treatment barriers at treated water venues, chlorination and filtration, might need to be expanded to include in-line (i.e., usually installed after filtration and before chlorination) supplemental disinfection (e.g., ultraviolet light irradiation, ozonation, or chlorine dioxide use). In-line supplemental disinfection can be used to improve the level of protection against pathogens, particularly Cryptosporidium. Improved monitoring of water-quality and facility maintenance programs and improved policies to educate the public and decrease body waste contamination of aquatic facilities should also reduce the risk for waterborne diseases. Because of the lack of protective barriers at swimming beaches, beach managers and public health officials should implement water-quality testing programs and educate swimmers concerning appropriate prevention measures, particularly measures addressing environmental pathogens unlikely to be prevented by current water-quality guidelines (e.g., illnesses caused by Vibrio and otitis media infections). Public health professionals should 1) improve training for pool inspectors, 2) update and improve pool codes to stay current with changing designs and needs demonstrated by outbreaks summarized in this report, and 3) lead the educational efforts with aquatic staff and the general public. Safe handling and use of chemicals at aquatic facilities needs to be taught and reinforced. In addition, to improve overall indoor air quality, public health professionals and pool managers need to understand the importance of indoor air quality so that improvements in pool water quality, swimmer hygiene, air-turnover rates, and ventilation will be implemented. Educating swimmers can play a vital role in reducing recreational water illness by instructing them to follow basic guidelines for healthy swimming. Fecal shedding of pathogens is common (43), so reducing the risk of water-related infection is best achieved by implementing diarrhea exclusion policies, using appropriate hygiene measures, and advising the public to minimize the swallowing of recreational water. ConclusionData collected by the WBDOSS are used to characterize the epidemiology of waterborne disease and outbreaks associated with both drinking and recreational water. Swimming is a common activity in the United States (44). Certain disease-causing agents are spread through shared bodies of water, and new waterborne pathogens that infect humans (e.g., Cryptosporidium and toxigenic E. coli) have emerged in the previous three decades. Recreational water illness and outbreaks are associated with both treated and untreated water and with every type of aquatic venue. Common themes derived from the outbreaks in this report include 1) low disinfectant levels, 2) inadequate water-quality monitoring, 3) high bather loads during large events, 4) breakdowns of equipment and lengthy detection times, 5) lack of essential cleaning of spas to minimize biofilm buildup, 6) accumulation of combined chlorines in pools accompanied by inadequate indoor air ventilation, 7) inadequately trained aquatic staff, 8) unclear communication chains for resolving problems, 9) outbreaks occurring on weekends when trained staff might be off duty, and 10) a lack of awareness by the general public of appropriate healthy swimming behaviors. Whereas no easy solution exists for reducing recreational water WBDOs, a sustained effort by the swimming public, the pool sector, and public health agencies can reduce the associated risk. The millions of persons in the United States who use recreational water every year can best reduce their risk by staying informed regarding the health and safety concerns associated with swimming. Public health officials should lead this educational effort to promote healthy swimming behaviors. Prevention methods discussed in this report should help make swimming experiences both safe and enjoyable. The aquatic sector also can benefit from the recommendations, which address changes that are needed in operation, maintenance, and chemical handling procedures. Large numbers of violations of state pool codes occur each year (14,31), indicating that improved pool operation, disinfection policies, and enforcement are needed to prevent recreational water illness (45). In addition, improvements in indoor air quality monitoring and widespread dissemination of validated testing protocols are needed to support improved air quality in indoor swimming pool settings. Public health professionals at all levels of government should lead a multidisciplinary approach to prevent recreational water illness that includes surveillance, health education, epidemiologic studies, laboratory support, and environmental health research. Educational resources and campaigns are needed for swimmers, parents, aquatic venue operators, and public health staff. Improved communications, particularly during outbreak investigations, between all levels of the public health system (e.g., infectious disease, environmental health, and surveillance staff) and between agencies in neighboring jurisdictions can 1) enhance awareness concerning ongoing occurrences of recreational water illness, 2) facilitate reporting to the WBDOSS in a more timely manner, and 3) strengthen WBDO investigations and responses to protect the public. The timely collection of clinical specimens and water samples for testing during a WBDO investigation and the initiation of an environmental investigation will result in more rapid identifications of the etiologic agent and determination of the conditions leading to the outbreak. However, the capacity of public health departments and laboratories to detect and investigate potential WBDOs varies and needs to be strengthened to meet these challenges. WBDO investigations typically require input from a variety of disciplines, including infectious disease epidemiology, environmental health, clinical medicine, water and sanitation engineering, and microbiology. Additional cross-training of existing personnel in these areas or additional staffing and resources are needed to improve WBDO detection, investigation, and reporting. CSTE passed a position statement at its 2006 annual meeting making waterborne disease outbreaks, as a unit of reporting, nationally notifiable and reportable to CDC starting in 2007. Adoption of this CSTE recommendation at the state level through state-specific legislative action might improve reporting of waterborne outbreaks at the state and local levels. CDC and EPA were also asked to develop training resources for WBDO investigations that are targeted to local and state/territorial public and environmental health workers responsible for WBDO detection, investigation, and reporting. In addition, CDC and EPA should collaborate with CSTE and developed national WBDO investigation and surveillance guidelines. The position statement is available at http://www.cste.org/PS/2006pdfs/PSFINAL2006/06-ID-12FINAL.pdf (Box). Acknowledgments The authors thank the following persons for their contributions to this report: state, local, and territorial waterborne-disease surveillance coordinators; state, local, and territorial epidemiologists and environmental health personnel; Mark Eberhard, PhD, Monica Parise, MD, John Williamson, PhD, Division of Parasitic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases (proposed), CDC; Lorraine Backer, PhD, Division of Environmental Hazards and Health Effects, National Center for Environmental Health, CDC; Charles Otto, Division of Emergency and Environmental Health Services, National Center for Environmental Health, CDC; Mohammed Siddiqui, New York State Department of Health, Troy, New York. References
* Composed of the Republic of the Marshall Islands, the Federated States of Micronesia, and the Republic of Palau; formerly parts of the U.S.-administered Trust Territory of the Pacific Islands. † Additional terms have been defined (Appendix A, Glossary of Definitions). Table 1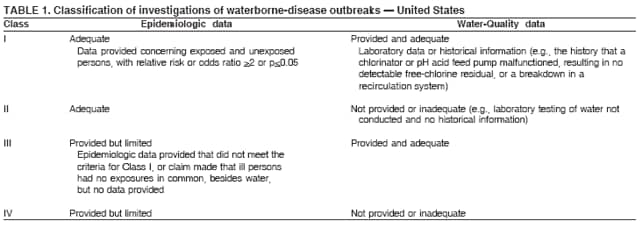 Return to top. Figure 1 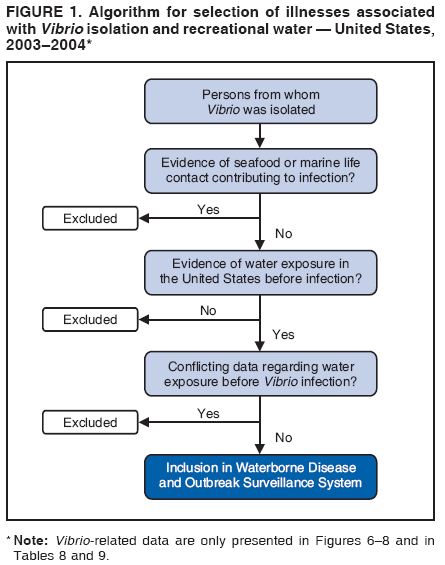 Return to top. Table 2 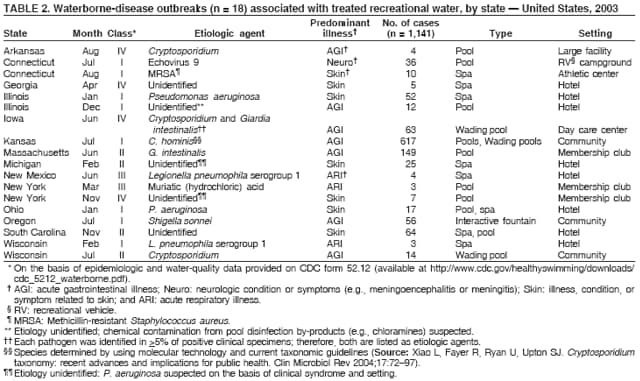 Return to top. Figure 2 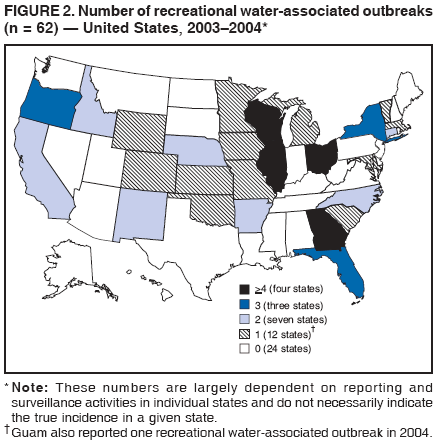 Return to top. Table 3 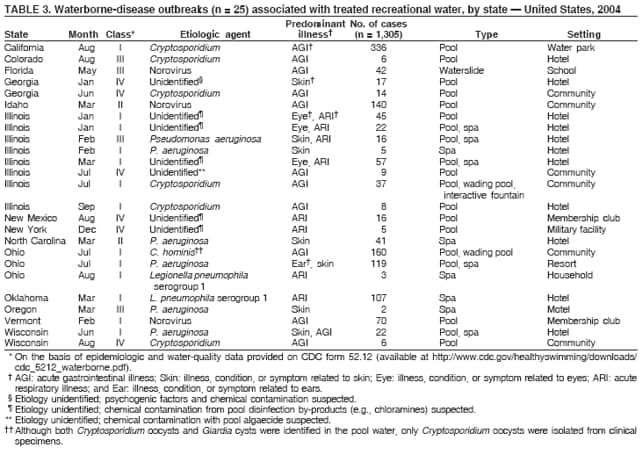 Return to top. Figure 3 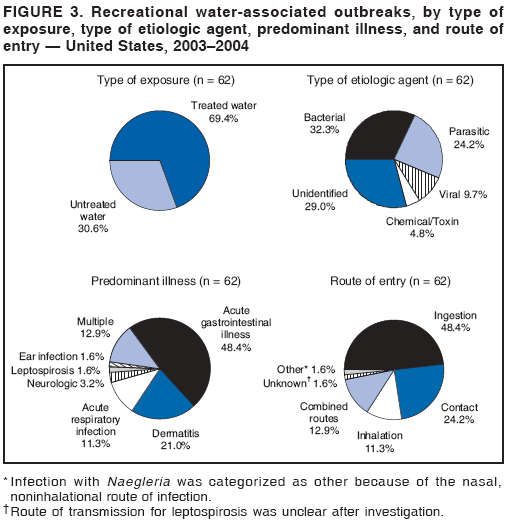 Return to top. Table 4 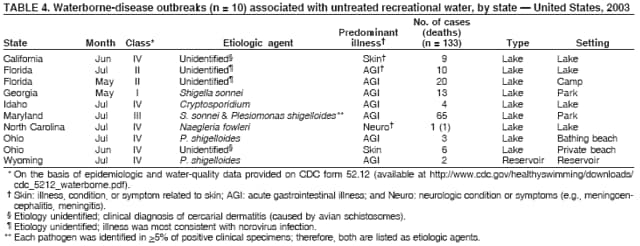 Return to top. Figure 4 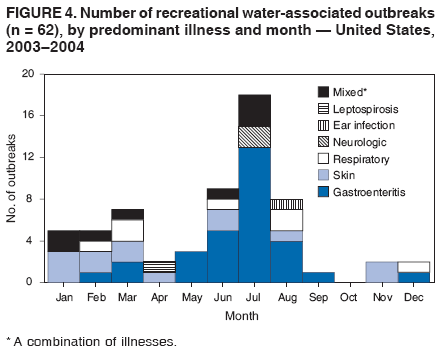 Return to top. Table 5 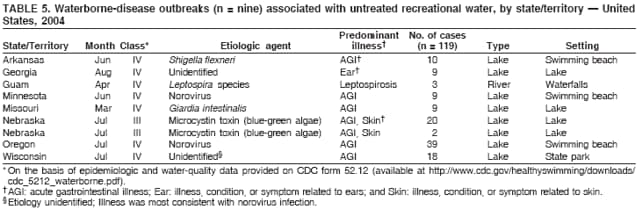 Return to top. Figure 5 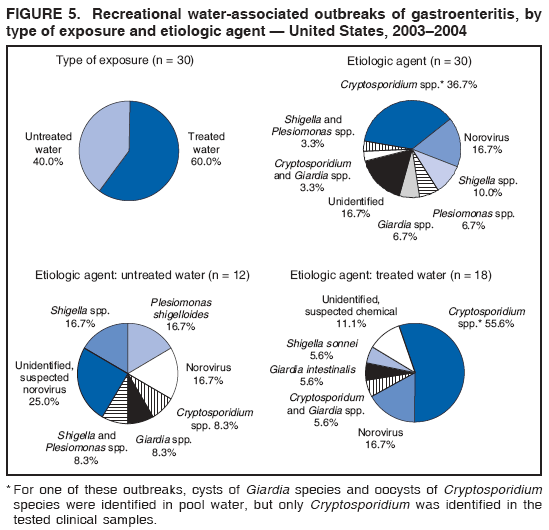 Return to top. Table 6 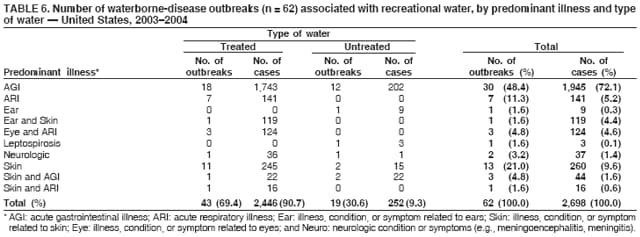 Return to top. Figure 6 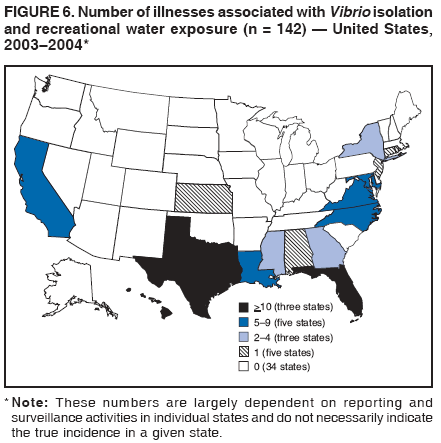 Return to top. Table 7 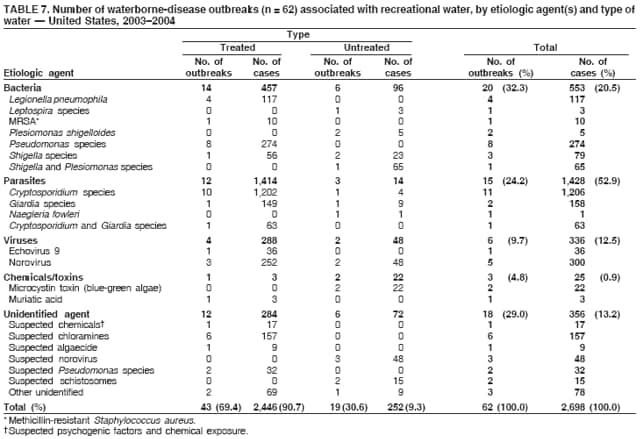 Return to top. Figure 7 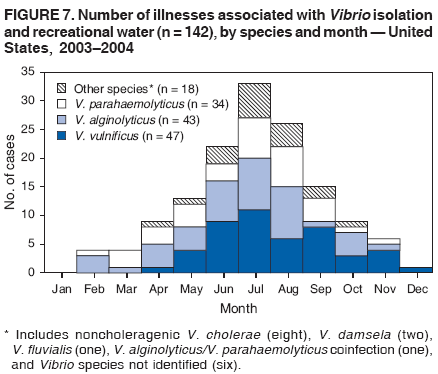 Return to top. Table 8 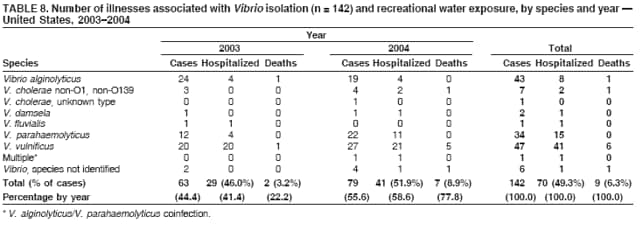 Return to top. Figure 8 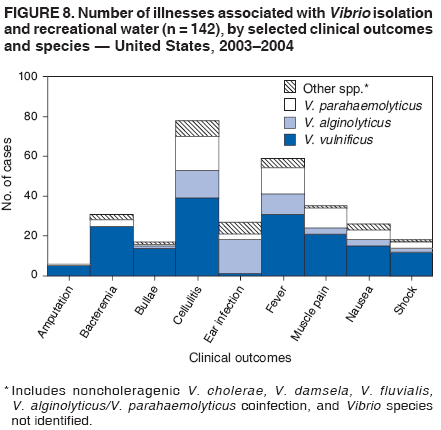 Return to top. Table 9 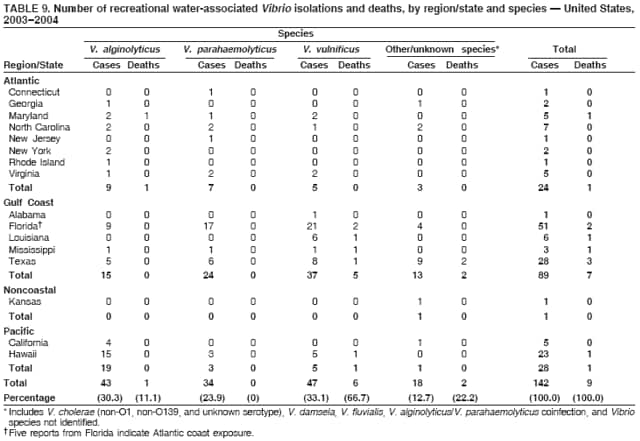 Return to top. Figure 9 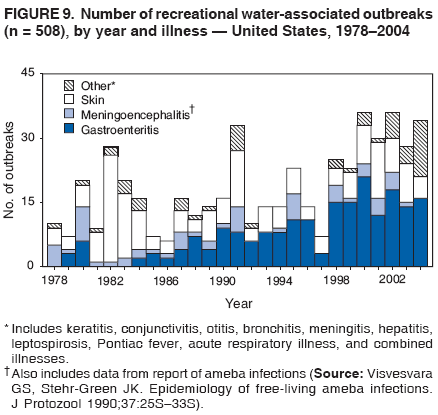 Return to top. Figure 10 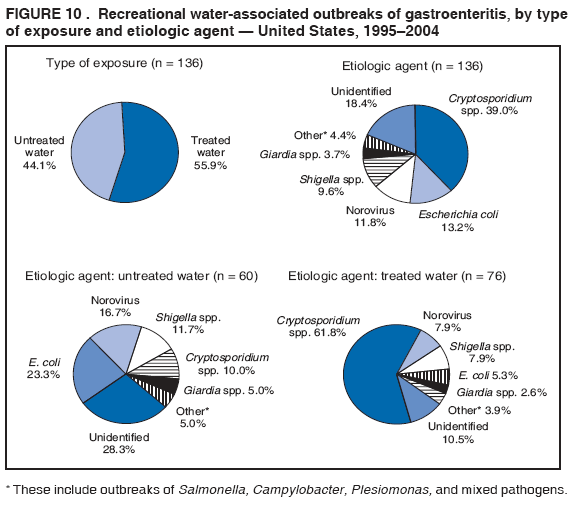 Return to top. Figure 11 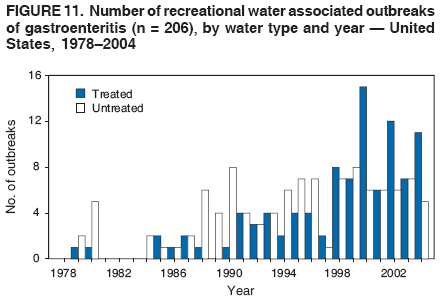 Return to top. Box  Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 12/11/2006 |
|||||||||
|