Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Bacterial Coinfections in Lung Tissue Specimens from Fatal Cases of 2009 Pandemic Influenza A (H1N1) --- United States, May--August 2009
On September 29, this report was posted as an MMWR Early Release on the MMWR website (http://www.cdc.gov/mmwr).
In previous influenza pandemics, studies of autopsy specimens have shown that most deaths attributed to influenza A virus infection occurred concurrently with bacterial pneumonia (1), but such evidence has been lacking for 2009 pandemic influenza A (H1N1). To help determine the role of bacterial coinfection in the current influenza pandemic, CDC examined postmortem lung specimens from patients with fatal cases of 2009 pandemic influenza A (H1N1) for bacterial causes of pneumonia. During May 1--August 20, 2009, medical examiners and local and state health departments submitted specimens to CDC from 77 U.S. patients with fatal cases of confirmed 2009 pandemic influenza A (H1N1). This report summarizes the demographic and clinical findings from these cases and the laboratory evaluation of the specimens. Evidence of concurrent bacterial infection was found in specimens from 22 (29%) of the 77 patients, including 10 caused by Streptococcus pneumoniae (pneumococcus). Duration of illness was available for 17 of the 22 patients; median duration was 6 days (range: 1--25 days). Fourteen of 18 patients for whom information was available sought medical care while ill, and eight (44%) were hospitalized. These findings confirm that bacterial lung infections are occurring among patients with fatal cases of 2009 pandemic influenza A (H1N1) and underscore both the importance of pneumococcal vaccination for persons at increased risk for pneumococcal pneumonia and the need for early recognition of bacterial pneumonia in persons with influenza.
CDC receives tissue specimens routinely from patients with confirmed or suspected infectious diseases and provides histopathologic, immunohistochemical, and molecular evaluations. Early in the 2009 influenza A (H1N1) virus pandemic, CDC provided guidelines for submission of tissue specimens for evaluation of influenza virus infections.* Confirmed fatal cases of 2009 pandemic influenza A (H1N1) were defined as influenza-like illness or postmortem findings suggestive of viral pneumonia and laboratory-confirmed 2009 pandemic influenza A (H1N1) virus infection by real time reverse transcriptase--polymerase chain reaction (rRT-PCR). Respiratory specimens (i.e., lung, trachea, and large-airway specimens) collected at autopsy were submitted to CDC by medical examiners, hospitals, and local and state health departments for additional evaluation.
Specimens were received from 77 patients who had 2009 pandemic influenza A (H1N1) virus infection confirmed before death (N = 41) or after death (N = 36). Of the 77 cases evaluated, 56 (72%) had at least some clinical information available, and 35 (45%) had preliminary autopsy reports submitted with the tissue specimens. All specimens were examined using hematoxylin and eosin stain, Lillie-Twort tissue Gram stain, and Warthin-Starry silver stain (Figure). Tissue specimens also were evaluated by various immunohistochemical assays using antibodies that are specifically reactive with S. pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, or Haemophilus influenzae. All bacteria were evaluated by a broad-range PCR assay that targets a segment of the 16S ribosomal DNA gene in DNA extracted from formalin-fixed, paraffin-embedded tissue (2). PCR for lytA and spy genes and pneumococcal serotyping by multiplex PCR were conducted to further characterize streptococcal coinfections.†
Of the 77 confirmed cases evaluated, 22 had histopathologic, immnohistochemical, and molecular evidence of coinfection with an identified bacteria, including 10 cases with S. pneumoniae, six with S. pyogenes, seven with S. aureus, two with Streptococcus mitis, and one with H. influenzae; four cases involved multiple pathogens (Table). The median age of the 22 patients was 31 years (range: 2 months--56 years); 11 (50%) were male. The cases were reported from eight states: California, Hawaii, Illinois, New Jersey, New York, Texas, Utah, and Virginia.
Duration of illness was available for 17 of the 22 patients; median duration was 6 days (range: 1--25 days). Fourteen of 18 patients with information available sought medical care while ill, and eight were hospitalized. Of the seven hospitalized patients with information available, all required mechanical ventilation. Seven of nine patients with information available on antimicrobial therapy were treated with antibiotics. Sixteen of the 21 patients for whom previous medical history was known had underlying medical conditions that were known to increase the risk for influenza-associated complications (16 patients) (3) or that were indications for vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV23) (15 patients).§
Reported by: J Louie, MD, C Jean, MPH, California Dept of Public Health. T-H Chen, MD, S Park, MD, R Ueki, Hawaii State Dept of Health. T Harper, MD, Stroger Hospital of Cook County; Chicago Dept of Public Health. E Chmara, MD, Northern Region Medical Examiner Office; New Jersey Dept of Health and Senior Svcs. J Myers, Erie County Medical Center; R Stoppacher, MD, Onondaga County Medical Examiner's Office; C Catanese, MD, Orange County Medical Examiner's Office; City of New York Office of the Chief Medical Examiner; New York City Dept of Health and Mental Hygiene; New York State Dept of Health. N Farley, MD, Valley Forensics, P.L.L.C.; Texas Dept of State Health Svcs. E Leis, MD, Utah Office of the Medical Examiner; Utah Dept of Public Health. C DiAngelo, MD, Northern District Office of the Chief Medical Examiner, Virginia; Virginia Dept of Health. AM Fry, MD, L Finelli, DrPH, Influenza Div, MG Carvalho, PhD, B Beall, PhD, M Moore, MD, C Whitney, MD, Div of Bacterial Diseases, National Center for Immunization and Respiratory Diseases; Infectious Diseases Pathology Br, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; DM Blau, DVM, PhD, EIS Officer, CDC.
Editorial Note:
During previous influenza pandemics, bacterial coinfections caused by S. pneumoniae, H. influenzae, S. aureus, and group A Streptococcus have been important contributors to morbidity and mortality (1,4). However, two early reviews of severe cases of 2009 pandemic influenza A (H1N1) showed no evidence of bacterial pneumonia among 30 hospitalized patients with laboratory-confirmed cases in California (5) and 10 intensive-care patients in Michigan (6). These reports might have led to a perception that bacterial coinfections are playing a limited role or no role in influenza deaths during the current pandemic. However, failure to document bacterial lung infections might reflect the difficulty of establishing specific bacterial diagnoses among persons with bacterial coinfections. Routine clinical tests used to identify bacterial infections among patients with pneumonia do not detect many of these infections. For example, <10% of patients who are hospitalized with clinically diagnosed pneumonia have blood cultures that are positive for bacterial infections (7). Histopathologic evaluation and testing of lung tissue, especially using PCR and immunochemistry methods, can detect many bacterial lung infections missed by standard clinical methods (2). The findings in this report indicate that, as during previous influenza pandemics, bacterial pneumonia is contributing to deaths associated with pandemic H1N1 and that histopathologic methods can be used to identify bacterial coinfections after death.
Although the findings in this report confirm the presence of bacterial lung coinfection, the results cannot be used to assess the prevalence of bacterial pneumonia among patients who have died from pandemic H1N1. The cases in this report do not come from a systematic sample and might not be representative of all pandemic H1N1 deaths or all pandemic H1N1 deaths associated with bacterial pneumonia. Systematic research is needed to determine the incidence and outcome of bacterial lung coinfections among patients with pandemic H1N1 virus infection and to quantify the role of these infections in fatal cases.
Medical examiners and coroners have an important role in the surveillance of deaths caused by the 2009 pandemic influenza A (H1N1) virus (8). Histopathologic techniques can assist with postmortem diagnosis of coinfections in patients in whom culture, antemortem or postmortem, does not detect bacteria. When autopsies are performed for patients with confirmed or suspected influenza who die after acute respiratory disease, a pathological evaluation of respiratory tissues should be conducted and should include testing for both viral and bacterial pathogens (8).
The findings in this report are subject to at least three limitations. First, not all potential bacterial pathogens (e.g., Legionella species) were evaluated. Second, the analysis of patient characteristics was based on limited patient information. Because medical records and death certificates generally were not available, no conclusion could be drawn about whether the cause of death was influenza, bacterial infection, or both. Third, because assessments of bacterial coinfections were conducted at autopsy, inadequate sampling, collection of specimens from unaffected portions of the lung, or prolonged illness and treatment before death might have prevented identification of bacteria.
The most common bacteria found in patients described in this report were S. pneumoniae. This infection was documented in 10 of the 22 patients. Although no data were available on the vaccination status of the 22 patients, one patient was aged <5 years and was therefore a candidate for pneumococcal conjugate vaccine, and 15 others had underlying medical conditions that were indications for PPSV23 vaccine (9,10). Persons at greatest risk for invasive pneumococcal disease include young children, older adults, and persons of any age with certain conditions, including chronic lung or cardiovascular disease and immunosuppressive conditions. All children aged <5 years should receive pneumococcal conjugate vaccine according to current Advisory Committee on Immunization Practices (ACIP) recommendations (9). In addition, PPSV23 is recommended for all persons aged 2--64 years with certain health conditions and all persons aged ≥65 years.¶ Available vaccination coverage data indicate that only a small proportion of persons aged 2--64 years in the United States who are recommended by ACIP to receive pneumococcal vaccine have received the vaccine. One study indicated that only 16% of persons aged 18--49 years with indications for PPSV23 vaccine had received the vaccine.** Because of the higher rates of 2009 pandemic H1N1 illness and death among persons aged 2--64 years, providers should target persons in this group who have existing ACIP indications for PPSV23 to receive the vaccine.
The findings in this report also underscore the importance of managing patients with influenza who also might have bacterial pneumonia with both empiric antibacterial therapy and antiviral medications.†† In addition, public health departments should encourage the use of pneumococcal vaccine, seasonal influenza vaccine, and, when the vaccine becomes available, pandemic influenza A (H1N1) 2009 monovalent vaccine.
References
- Morens DM, Taubenberger, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008;198:962--70.
- Guarner J, Packard MM, Nolte KB, et al. Usefulness of immunohistochemical diagnosis of Streptococcus pneumoniae in formalin-fixed, paraffin-embedded specimens compared with culture and gram stain techniques. Am J Clin Pathol 2007;127:612--8.
- CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-8).
- Brundage JF, Shanks GD. Deaths from bacterial pneumonia during the 1918--19 influenza pandemic. Emerg Infect Dis 2008;14:1193--9.
- CDC. Hospitalized patients with novel influenza A (H1N1) virus infection---California, April--May, 2009. MMWR 2009;58:536--41.
- CDC. Intensive-care patients with severe novel influenza A (H1N1) virus infection---Michigan, June 2009. MMWR 2009;58:749--52.
- Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med 2004;169:342--7.
- Nolte KB, Lathrop SL, Nashelsky MB, et al. "Med-X": a medical examiner surveillance model for bioterrorism and infectious disease mortality. Hum Pathol 2007;38:718--25.
- CDC. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2000;49(No. RR-9).
- CDC. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1997;46(No. RR-8).
* Additional information available at http://www.cdc.gov/h1n1flu/tissuesubmission.htm.
† Additional information available at http://www.cdc.gov/ncidod/biotech/strep/protocols.htm.
§ Additional information available at http://www.cdc.gov/h1n1flu/guidance/ppsv_h1n1.htm.
¶ Additional information available at http://www.cdc.gov/h1n1flu/guidance/ppsv_h1n1.htm.
** Additional information available at http://www.cdc.gov/flu/professionals/vaccination/pdf/NHIS89_07ppvvaxtrendtab.pdf.
†† Additional information available at http://www.cdc.gov/h1n1flu/recommendations.htm.
FIGURE. Histochemical and immunohistochemical diagnosis of Streptococcus pneumoniae infection in a patient with confirmed 2009 pandemic influenza A (H1N1). (A) Detection of Gram-positive cocci (arrows) with use of Lillie- Twort Gram stain of lung tissue (original magnification ×63). (B) Immunohistochemical staining of multiple S. pneumoniae (arrows) with use of immunoalkaline phosphatase with naphthol-fast red and hematoxylin counterstain (original magnification ×63).
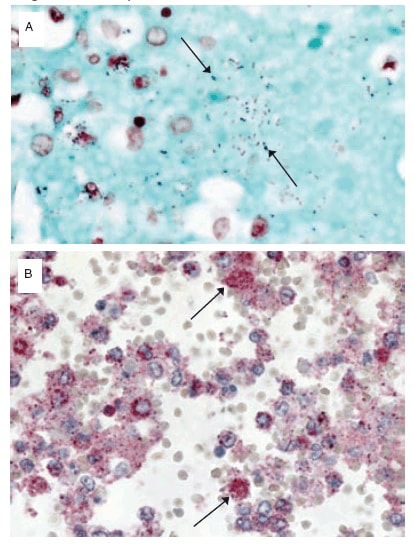
Alternative Text: The Figure above consists of two slides showing Streptococcus pneumoniae infection in a patient with confirmed pandemic H1N1 virus infection.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 10/1/2009


