

|

|

Volume 5:
No. 4, October 2008
ORIGINAL RESEARCH
Exploring the Feasibility of Combining Chronic Disease Patient Registry Data to Monitor the Status of Diabetes Care
Angela M. Kemple, MS, Noelle Hartwick, RN, MPH, Marilyn H. Sitaker, MPH, Jeanne J. Harmon, RD, MBA, CDE, Kathleen Clark, MS, RD, CDE, Jan Norman, RD, CDE
Suggested citation for this article: Kemple AM, Hartwick N, Sitaker MH, Harmon JJ, Clark K, Norman J. Exploring the feasibility of combining chronic disease patient registry data to monitor the status of diabetes care. Prev Chronic Dis 2008;5(4).
http://www.cdc.gov/pcd/issues/2008/
oct/07_0156.htm. Accessed [date].
PEER REVIEWED
Abstract
Introduction
To provide direction and to support improvements in diabetes care, states must be able to measure the
effectiveness of interventions and gain feedback on progress. We wanted to know if data from multiple health clinics that are implementing quality improvement strategies could be combined to provide useful measurements of diabetes care processes and control of intermediate outcomes.
Methods
We combined and analyzed electronic patient health data from clinic sites across Washington State that used the Chronic Disease Electronic Management System (CDEMS)
registry. The data were used to determine whether national and state objectives for diabetes care
were met. We calculated the percentage of patients that met standards of care in 2004.
Results
The pooled dataset included 17,349 adult patients with diabetes from 90 clinics. More than half of patients were above recommended target levels for hemoglobin A1c testing, foot examination, hemoglobin A1c control, and low-density lipoprotein cholesterol control. Fewer patients met recommendations for nephropathy assessment, eye examinations, and blood pressure control. In terms of meeting these standards, rates of diabetes care varied across clinics. CDEMS rates of care were compared with
those reported by other data sources, but no consistent pattern of similarities or differences emerged.
Conclusion
With committed staff time, provider support, and resources, data from clinical information systems like CDEMS can be combined to address a
deficiency in state-level diabetes surveillance and evaluation systems — specifically, the inability to capture clinical biometric values to measure intermediate health outcomes. These data can complement other surveillance and evaluation data sources to help provide a better picture of diabetes care in a state.
Back to top
Introduction
Diabetes is a growing public health problem (1), but care
continues to be less than optimal (2-4), despite evidence that effective and
economical interventions can result in fewer complications and improved outcomes
(5-7). Therefore, the Institute of Medicine labeled diabetes as a
priority area for quality improvement in the United States (8) and suggested
substantial changes in and redesign of health care systems (9), including the
better use of information technology to monitor health care.
National and state objectives for diabetes care are used to evaluate the
effectiveness of prevention and intervention activities (10). Local targets for these objectives have been set by each state’s Diabetes Prevention and Control Program (DPCP). Objectives for the Washington State DPCP focus on increasing population-level rates of process measures (annual foot and eye examinations, biannual hemoglobin A1c
[HbA1c] tests, annual nephropathy
assessment, annual influenza vaccination, and previous pneumococcal vaccination)
and intermediate outcomes (controlled levels of HbA1c, blood pressure, and
low-density lipoprotein [LDL] cholesterol).
States must be able to measure the cumulative effect of broad community, health system, and health communication interventions and
to monitor progress toward diabetes objectives over time. Aggregate data from individual clinical information systems have been used by large organizations, including health care systems, federal health care organizations, and
community health centers to monitor, coordinate, and manage care for targeted diabetes populations (11-22). State health departments,
however, have used these kinds of data to only a limited degree (23). The lack of state-specific surveillance data for measuring progress on
3 diabetes indicators — glucose, lipid, and blood pressure control —
is a deficiency in evidence supporting the impact of a state health department’s effort to improve diabetes outcomes.
In 2005, the Washington State DPCP assessed its progress toward meeting state and national diabetes objectives to determine whether established targets for each objective needed to be modified. An extensive review of population-based data was done to ascertain what data sources could be incorporated into the surveillance program to help track progress toward meeting objectives.
Whereas the DPCP regularly uses statewide BRFSS (Behavioral Risk Factor Surveillance System)
telephone survey data to monitor processes of care (foot examination, eye
examination, HbA1c testing,
and influenza and pneumococcal vaccinations), no statewide source exists for collecting information on nephropathy screening and intermediate health outcomes (glucose, lipid, and blood pressure control). For this reason, the DPCP decided to explore the feasibility of obtaining data from a patient registry known as the Chronic Disease Electronic Management System (CDEMS),
which is used by primary care
providers across Washington (24). CDEMS is the only source of state-specific data available to the DPCP to monitor nephropathy screening and HbA1c, LDL, and blood pressure values among a large patient population with diabetes. Consolidated data from all Washington clinics using CDEMS covered
approximately 13% of all state residents with diabetes in 2004.
Our goals for this data consolidation project were to 1) measure the status of patients in CDEMS registries in terms of meeting state and national objectives for diabetes, 2) provide aggregate comparison data for individual clinics using CDEMS, and 3) determine the feasibility of combining and using clinic data for ongoing diabetes surveillance and evaluation efforts in Washington. This report focuses on the
process of aggregating registry data, resources used, initial outcomes, lessons learned, and the utility of combining clinic data for future endeavors.
Back to top
Methods
DPCP program staff, Washington State Department of Health (DOH) project epidemiologists, and 2 contractors worked together to plan, coordinate collection of, consolidate, and cleanse CDEMS data. The DPCP contracted with the CDEMS technical support consultant and with Krupski Consulting, Inc, Olympia, Washington, a firm that
extracts, transforms, and loads data
(25). The Appendix provides details on the tasks, estimated time, and cost for this project.
At the start of this project, epidemiologists spent considerable time working with DPCP staff and the CDEMS technical consultant to gain a thorough understanding of the development philosophy, implementation, maintenance, and structure of CDEMS and variations between clinics to guide clinic recruitment and subsequent data consolidation. CDEMS is a Microsoft Access database application developed by the Washington State DPCP in 2002 (24). It is available at no cost to all who wish to use
it (http://www.cdems.com). The program was designed to help medical providers, clinic managers, and other health care staff track the care of patients with chronic health conditions. CDEMS stores individual patient demographic information, visit dates, vital signs, medications, diagnoses, services, laboratory results, and custom notes in
7 main data tables. Data entry screens, such as the Patient Information Record and New Visit Form (Figure), are used to populate the main tables in the
database. The CDEMS registry has predefined data codes to track diabetes care, but these can be modified and measures added to monitor other chronic conditions. A laboratory interface is available to download results from several major laboratories electronically. Printed progress notes, patient lists, and summary reports are generated from the registry database to help deliver services more efficiently and effectively and to monitor changes from quality improvement efforts.
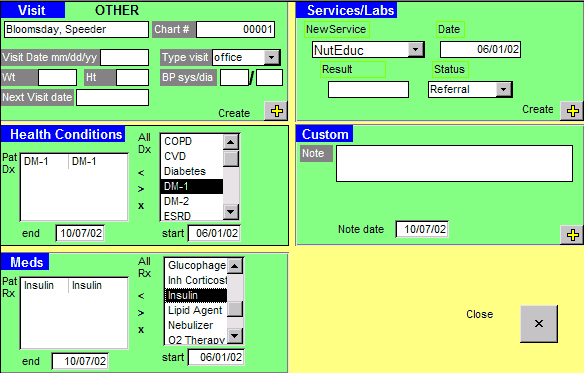
Figure. New Visit Form With Example Patient Data,
Chronic Disease Electronic Management System, Washington State Department of
Health, 2002. Published with permission.
CDEMS registries are used predominately in primary care clinics in community and rural settings and in Indian Health Service clinics throughout the state. Most clinics began using CDEMS as part of the Washington State Collaborative (14,26) or National Health Disparities Collaborative (27). These collaboratives use a proactive approach that offers proven strategies to help primary care practice teams manage care for people with chronic diseases. For this reason, we considered most patients in
CDEMS to be potentially better managed than the general diabetes population in Washington state.
Clinics populated their registries by either entering data on new patients prospectively, entering data from review of medical records retrospectively, or importing data from other systems. Some clinics populated their registries with a subset of their patients,
whereas others used their total diabetes patient population. Demographic data were often imported from a billing system and were complemented by data abstracted from medical records, to capture recent and historical health information
going back 1 to 2 years. At the time of this project, only 22% of clinics used laboratory interfaces to download laboratory results electronically.
Because of variations in how clinics used data fields in CDEMS, we compiled a master list of data fields associated with each table in the registry, which included only those that would be useful for measuring progress toward state and national objectives.
Because CDEMS stores individually identifiable health information, protection under the Health Insurance Portability and Accountability Act (HIPAA) was reviewed by the HIPAA officer for
the Washington State DOH. The project was designated as public health surveillance and considered to be in compliance with HIPAA privacy rule requirements (28).
The Washington State DOH Information Technology Security Office conducted an
assessment of data confidentiality and sensitivity before clinic recruitment and data aggregation.
To minimize the data transfer burden and encourage response, we asked clinics to simply copy their registry database, including all patient records, data fields, and years available, onto a CD-ROM, DVD, or floppy disk and send it directly to the DPCP in a return postage-paid mailing envelope. We recruited clinics by e-mail initially, followed up with a formal letter explaining the project, and contacted nonresponding clinics by e-mail and telephone. Clinics that participated in the project were later provided a summary of results from the combined CDEMS database to compare with their own data.
Collecting, combining, and cleansing data
The CDEMS technical support contractor coordinated the collection and transfer of databases from the clinics to the DPCP. DPCP staff logged clinics’ CDEMS databases by date of arrival before forwarding data to Krupski Consulting for consolidation. This contractor worked closely with project staff and the epidemiologists to identify the relevant CDEMS variables and data fields needed to assess the number of patients meeting state and national objectives. Clinics with multiple locations
submitted data inconsistently, some providing a database for each location and others combining locations into
1 large database. In addition, some clinics included only a subset of their total patient population. As data were received, project staff created a unique code to identify the source of data.
The aim of data consolidation was to combine the records from source tables
in each clinic’s registry into a single master database containing values with
consistent meaning. Because CDEMS was designed to be adaptable to each clinic,
clinics entered data in various ways, and thus transforming and cleansing the data before aggregation was time-consuming. Values for each field in the CDEMS database were standardized to reconcile variations between clinics. For example, glucose
in the original datasets received from each clinic may have been recorded as Glu, GLU, glu, glucose, or Glucose; all terms were changed to glucose in the aggregate database. In addition, the contractor ensured that codes predefined in the original databases for diagnosis, service, and laboratory fields were incorporated into the aggregate database and dealt with formatting issues.
To make subsequent data analysis more efficient, the contractor worked with project staff and the epidemiologists to combine and recode similar values. For example, all insurance plan names in the database were recoded to 5 unique values — commercial/private, Medicaid, Medicare, self-pay, or no insurance. Some field values were automatically assigned,
but others had to be done manually, case-by-case. Unknown or invalid values were set to null in the condensed database. To determine
health conditions alone, we examined and recoded more than 130 different data field values. More than 200 different field values were reviewed and recoded for health services, more than 500 values for laboratory results, and more than 2,000 values for health care coverage. The contractor added new fields to various tables in the combined database to document how original values were recoded.
The contractor applied proprietary software tools to consolidate data efficiently and cost-effectively. Afterward, the epidemiologists reviewed data, removed duplicate databases, and organized and linked data tables for analysis. Following these activities, 493 records (<1%) were excluded from further analysis.
Diabetes was recorded in the CDEMS table that stores information on a
patient’s health conditions by clinic staff if a patient had an International
Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM)
diagnosis code of diabetes type 1 or type 2 (confirmed through chart audit) and
a date of diagnosis. For example, if a patient had diabetes, a provider would
select a preset health condition code for diabetes (Diabetes, DM-1, or DM-2)
under the health conditions section in the CDEMS New Visit Form (Figure). For our analysis, we selected adult patients with diabetes who met the following criteria: 1) had at least 1 visit, service, or laboratory result in 2004, 2) were diagnosed with diabetes before 2004 so they had a full year to receive services, and 3) were at least 18 years old. Patients with gestational diabetes or prediabetes were excluded. The final pooled CDEMS database included 51,233 patients, of which 17,349 met these
criteria.
Measures
We were able to adequately assess the indicators described in
Table 1, which lists the indicator definitions and reporting ranges we selected
before analysis. We were unable to assess receipt of annual influenza vaccine and previous pneumococcal vaccination because patients are usually referred to other facilities for vaccinations, and few clinics have a feedback system to monitor outside services. Further, most clinics did not collect historical information on lifetime pneumococcal
vaccination.
Statistical analysis
The data were analyzed using Microsoft Access 2003 (Microsoft Corp, Redmond, Washington) and Stata statistical software, version 9.0 (StataCorp LP, College Station, Texas). Percentages and 95% confidence intervals were calculated using the binomial Wald method. Median percentages and ranges across clinics were also calculated because of substantial variation in rates of meeting diabetes care objectives among clinics.
Back to top
Results
Most of the 132 eligible Washington clinics (85%) submitted data for this project. More than two-thirds (68%) provided data that were included in final sample of 17,349 adult patients with diabetes from 90 primary care office settings. Clinics in the combined database ranged in size from 1 to 2,483 patients with diabetes in 2004. More than 90% of clinics participated in a collaborative or were a satellite clinic of an organization that participated in a collaborative. Approximately 40% were
community health centers or federally qualified community health centers.
We excluded data from 22 clinics (17%) from our analysis because the clinic database could not be opened or combined, the clinic’s registry was not implemented at the start of the project period, or the clinic’s data collection and reporting methods made it difficult to identify diabetes patients. No particular pattern was noted with the information available from these clinics compared with that from clinics included in the combined database.
Twenty clinics (15%) did not participate in the project because they did not start their registry until late in 2004, had staff turnover involving the CDEMS coordinator at the clinic, or lacked time. We had insufficient information to compare patient populations between CDEMS clinics that submitted data and CDEMS clinics that did not. The clinics that did not participate came mostly from nonurban areas, but we observed no further differences such as private
vs public status,
participation
in the Washington State Collaborative, or geographic location.
The average age of patients with diabetes in the combined database was 59 years (range 18-100 years).
Slightly more than half (53%) were female. Race/ethnicity was documented for only 60% of patients, 59% of whom were listed as white, 21% Hispanic, 8% African American, 6% Asian, 3% American Indian/Alaska Native, 2% Native Hawaiian/Other Pacific Islander, and
less than 1% other race.
Approximately 38% of the participants were commercially insured, 22% had Medicare, 19% had unknown insurance status, 8% had no
health insurance, 9% had Medicaid, 5% were self-pay, and less than 1% had other sponsored care.
The age and sex distribution of adult patients with diabetes in the CDEMS database was different from that of the overall Washington adult diabetes population
(Table 2). A larger
proportion of CDEMS patients with diabetes were aged 65-74 years and a smaller
proportion were aged 75 years or older compared with the overall population, and
the proportion of women was greater among CDEMS patients. Hispanics and Asians appeared to be overrepresented in CDEMS compared with the state. However,
because the race and
ethnic origin of many patients was not recorded in CDEMS, we are unable to draw conclusions about the differences between these populations. Similarly, the large proportion of CDEMS patients listed as having “unknown insurance status” means that we are unable to comment on differences in health insurance coverage between CDEMS and the overall statewide populations.
Tables 3 and
4 show the distribution of processes of diabetes care and intermediate health outcomes among adult patients with diabetes in the aggregate database. More than 50% of patients were above recommended target levels for HbA1c testing, foot examination, HbA1c control, and LDL cholesterol control. Fewer patients met recommendations for nephropathy assessment, eye examinations, and blood pressure control. Performance on these indicators varied across clinics.
Table 5 further describes
the values for each of the intermediate health outcomes assessed.
Table 6 compares results from the consolidated CDEMS database with results from other state and national data sources. CDEMS patients had more favorable results for HbA1c and LDL cholesterol levels than
did the overall population, and their results did not differ noticeably for receiving an HbA1c test, LDL cholesterol test, or nephropathy screen in the past year. CDEMS results were less favorable for annual foot examinations, annual eye examinations, and biannual HbA1c, compared with other data
sources.
Back to top
Discussion
We sought to determine the feasibility of using aggregate clinic data for ongoing diabetes surveillance and evaluation efforts in Washington State. Our work shows that with committed staff time, provider support, and resources, data from clinical information systems like CDEMS can be combined to address a
deficiency in state surveillance and evaluation systems — specifically, the inability to capture clinical values to measure intermediate health outcomes for diabetes. The intent
is to use the CDEMS measures that do not appear in BRFSS to complement BRFSS data, with the understanding that one of the limits of the aggregate CDEMS database is that it reflects only 9% of the diabetes population — and they are probably specially managed because most clinics have received intensive training on implementing the Chronic Care Model (29,30).
CDEMS patients’ better HbA1c and LDL cholesterol levels compared with the overall state population of people with diabetes (Table 6) may be because most CDEMS clinics were alumni of the Washington State Collaborative (14,26) or Health Disparities Collaborative (27) or were affiliated with a clinic that participated in a collaborative that focused on these measures.
CDEMS screening results for HbA1c, LDL cholesterol, or nephropathy in the past year were not noticeably different compared with data sources that were not restricted to specially managed populations.
The CDEMS results also were not as good for annual foot examinations, annual eye examinations, and biannual HbA1c tests.
Some differences between the CDEMS results and other data sources could be attributable to differences in how measures are defined, how ranges for responses are defined, how data are collected (clinical data vs self-report), and how data are entered into systems,
or they may represent a true difference in outcomes. For example, the low
prevalence of receiving eye examinations in CDEMS compared with self-reported eye examination data from statewide BRFSS is not unexpected. Follow-up documentation on
patients referred for eye examinations outside the care clinic is rare, and poor agreement between self-report and medical record data on annual eye examinations has been documented elsewhere (31). Without a detailed study comparing the data sources, clinics, patients
who are captured within the data sources, or study methods, it is difficult to explain observed differences.
Lessons learned from combining data
A project of this magnitude required a commitment from the state DPCP to
ensure that financial and staff resources were available to complete the work.
The project required substantial time, coordination, and communication from
internal and external staff who assisted with project management and clinic recruitment; contractors and programmers, who managed data submission and consolidation; CDEMS staff, who provided technical support; and epidemiologists, who provided
project coordination, project design, data consultation, and analysis.
Clinic recruitment was facilitated by established relationships between DPCP and CDEMS users through the ongoing technical assistance provided to clinics by DPCP as part of the Washington State Collaborative. We learned to work with the clinics’ central registry coordinators (especially for multisite implementations) rather than each clinic within a larger system. It was also necessary to be explicit about which project we represented, since multiple quality
improvement evaluation projects occurred simultaneously within the Washington State DOH.
We found we needed to provide several options for ensuring patient privacy and data security with the clinics and Washington State DOH information technology staff before arriving at a simple and acceptable process for gathering data. Although we initially favored a secure file transfer protocol Web site as a central repository for data submission, this option would have caused undue burden, compromising clinic participation. Even with the easier option of copying the databases to a CD,
several clinics needed our assistance to transfer their data.
During data consolidation, we needed to complete several tasks to analyze data more efficiently (eg, reviewing various field codes from each clinic, combining similar values, handling different types of data, and identifying data fields to define measures). After data were combined, more time was spent identifying unique patients and removing duplicate data. Resolving duplications first may have minimized postconsolidation cleanup of the data and ensured accuracy of numerator and
denominator counts required to estimate the percentage receiving care. It would have been useful to have a comprehensive codebook before analysis to identify field names and values, track programming used to combine data, and note changes made to the original data submitted.
After data were combined and reviewed, we still needed to modify some project measures. For example, because there is no standard method for reporting nephropathy results in CDEMS, we reviewed results manually to determine which met the definition for annual screening. The distinctive ability for the user to customize CDEMS led to variation in the data that required additional effort on our part to standardize before analysis.
Limitations
Our project had several limitations. First, it may be biased toward better outcomes because participating clinics are engaged in quality improvement efforts, although quality improvement efforts may have focused on a few measures only, and specialty clinics are generally not represented in CDEMS. Second, in this initial look at the data, only unweighted aggregate population statistics are reported; thus, clinics with larger patient populations may disproportionately affect the results. Our
combined data represent a convenience sample, and detailed information to construct sample weights was not available. In the future, additional time and resources will be required to collect detailed information on each clinic and account for differences between clinics. Third, individual clinic datasets reflect variations in entry protocols, reporting methods, field definitions, and years covered. Some clinics collected registry data for a few objectives during the project period. Our overall
rates may have been higher had we been able to account for inconsistencies in data collection intensity and measurement standards across clinics. Because we were unable to
identify new patients based on the start date field in CDEMS (ie, clinics used different definitions for “start date”), our results may include patients who did not participate throughout the entire project period. These limitations highlight the need for improved standards in CDEMS data collection and reporting.
Implications
This project reflects the status of state and national objectives for approximately 9% of adults with diabetes in Washington in 2004. However, the number of patients tracked in CDEMS grew by 83% from 2004 to 2007 (32). Furthermore, provider use of CDEMS grew by 39% during the same period (32). As more providers use clinical information systems like CDEMS, the potential to gather more representative data will
improve. This data quality will be necessary as provider accountability,
pay-for-performance, and public reporting of quality measures are increasingly emphasized. How the growing use of electronic medical records (EMRs) will influence CDEMS use has yet to be determined, but some clinics in Washington use both CDEMS and EMR registries (17% in 2007) because of the limited usefulness of EMR systems to track patients with chronic conditions (32,33).
This project shows there is a need to improve standardization of CDEMS data entry and reporting for a minimum number of key measures to track progress over time, to provide appropriate and valid comparison data, and to
help organizations to share knowledge about progress with one another. For example, establishing a consistent feedback loop and data controls to capture completed referrals for eye examinations would improve monitoring of this objective in the population.
Conclusion
This project shows how one state combined individual clinic data from chronic disease registries as part of an overall effort to enhance its diabetes surveillance capacity. Being able to monitor the status of diabetes care, track changes, and conduct peer comparisons through the collection, combination, and use of clinic data may help stimulate health practitioners to implement broad systematic improvements and provide data to the DPCP for future program plans.
Back to top
Acknowledgments
The authors thank the following for supporting this project: the clinicians and staff from each of the participating primary care practices that submitted their CDEMS data; Dusty Knobel and Jackie Gianunzio for their CDEMS technical support and advisement; Eric Ossiander for help with project development and data analysis; Krupski Consulting, Inc, for consolidation of the CDEMS data; George Zipf and operations staff for
data from the National Health and Nutrition Examination Survey; James Peterson and staff at Quest Diagnostics, Inc, for
providing information on valid reporting values and edit filters; Amy Knutz-Gibson for support with data collection; and Francisco Arias-Reyes for organizing dissemination of results.
This project was supported through cooperative agreement U32/DP022172-4 between the Washington State Department of Health, DPCP, and the Centers for Disease Control and Prevention, Division of Diabetes Translation.
Back to top
Author Information
Corresponding Author: Angela M. Kemple, MS, Epidemiologist, Chronic Disease Prevention Unit, Washington State Department of Health, PO Box 47855, Olympia, WA 98504-7855. Telephone: 360-236-3652. E-mail:
angela.kemple@doh.wa.gov.
Author Affiliations: Noelle Hartwick, Marilyn H. Sitaker,
Jeanne J. Harmon, Jan Norman, Chronic
Disease Prevention Unit, Diabetes Prevention and Control Program, Washington State Department of Health, Olympia, Washington. Kathleen Clark, Health Care Authority, Olympia, Washington. At the time of this project, Ms Clark was manager of the Washington
State Diabetes Prevention and Control Program.
Back to top
References
- Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al.
The evolving diabetes burden in the United States. Ann Intern Med 2004;140(11):945-50.
- Centers for Disease Control and Prevention.
Prevalence of receiving multiple preventive-care services among adults with diabetes — United States, 2002-2004. MMWR Morb Mortal Wkly Rep 2005;54(44):1130-3.
- Mukhtar Q, Jack L Jr, Martin M, Murphy D, Rivera M. Evaluating progress toward Healthy People 2010 national diabetes objectives. Prev Chronic Dis 2006;3(1).
http://www.cdc.gov/pcd/issues/2006/jan/05_0122.htm. Accessed June 13, 2007.
- Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, et
al.
Improvements in diabetes processes of care and intermediate outcomes: United States, 1988-2002. Ann Intern Med 2006;144(7):465-74.
-
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329(14):977-86.
- Litzelman DK, Slemenda CW, Langefeld CD, Hays LM, Welch MA, Bild DE, et
al.
Reduction of lower extremity clinical abnormalities in patients with non-insulin-dependent diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1993;119(1):36-41.
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al.
Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321(7258):405-12.
- Adams K, Corrigan JM, eds. Priority areas for national action: transforming health care quality. Washington (DC): National Academies Press; 2003.
- Committee on Quality of Health Care in America: Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington (DC): National Academies Press; 2001.
- Safran MA, Mukhtar Q, Murphy DL.
Implementing program evaluation and accountability for population health: progress of a national diabetes control effort. J Public Health Manag Pract 2003;9(1):58-65.
- Acton KJ, Shields R, Rith-Najarian S, Tolbert B, Kelly J, Moore K, et al.
Applying the diabetes quality improvement project indicators in the Indian Health Service primary care setting. Diabetes Care 2001;24(1):22-6.
- Benedetti R, Flock B, Pedersen S, Ahern M.
Improved clinical outcomes for fee-for-service physician practices participating in a diabetes care collaborative. Jt Comm J Qual Saf 2004;30(4):187-94.
- Coppell KJ, Anderson K, Williams S, Manning P, Mann J.
Evaluation of diabetes care in the Otago region using a diabetes register, 1998-2003. Diabetes Res Clin Pract 2006;71(3):345-52.
- Daniel DM, Norman J, Davis C, Lee H, Hindmarsh MF, McCulloch DK, et al.
A state-level application of the chronic illness breakthrough series: results from two collaboratives on diabetes in Washington state. Jt Comm J Qual Saf 2004;30(2):69-79.
- Georgiou A, Burns J, McKenzie S, Penn D, Flack J, Harris MF.
Monitoring change in diabetes care using diabetes registers — experience from divisions of general practice. Aust Fam Physician 2006;35(1-2):77-80.
- Harwell TS, McDowall JM, Gohdes D, Helgerson SD, Montana Diabetes Health Center Team.
Measuring and improving preventive care for patients with diabetes in primary health centers. Am J Med Qual 2002;17(5):179-84.
- Hupke C, Camp AW, Chaufournier R, Langley GJ, Little K. Transforming diabetes health care: part 2.
Changing lives. Diabetes Spectr 2004;17:107-11.
- Johnson EA, Webb WL, McDowall JM, Chasson LL, Oser CS, Grandpre JR, et al. A field-based approach to support improved diabetes care in rural states. Prev Chronic Dis 2005;2(4).
http://www.cdc.gov/pcd/issues/2005/oct/05_0012.htm. Accessed June 13, 2007.
- Joshy G, Simmons D.
Diabetes information systems:
a rapidly emerging support for diabetes surveillance and care. Diabetes Technol Ther 2006;8(5):587-97.
- MacLean CD, Littenberg B, Gagnon M.
Diabetes decision support: initial experience with the Vermont diabetes information system. Am J Public Health 2006;96(4):593-5.
- McCulloch DK, Price MJ, Hindmarsh M, Wagner EH.
A population-based approach to diabetes management in a primary care setting: early results and lessons learned. Eff Clin Pract 1998;1(1):12-22.
- Skinner A, Fraser-Maginn R, Mueller KJ.
Chronic disease management systems registries in rural health care. Rural Policy Brief 2006;11(1 (PB2006-1)):1-8.
- Desai J, Geiss L, Mukhtar Q, Harwell T, Benjamin S, Bell R, et al.
Public health surveillance of diabetes in the United States. Public Health Manag Pract 2003;Suppl:S44-51.
- The CDEMS user network. Olympia (WA): Washington State Department of
Health, Diabetes Prevention and Control Program; 2002. http://www.cdems.com/.
Updated June 8, 2007. Accessed June 13, 2007.
- Krupski consulting. Olympia (WA): Krupski Consulting, Inc. http://krupski-consulting.com/. Accessed June 13, 2007.
- Washington State Collaborative. Olympia (WA): Washington State Department of Health, Diabetes Prevention and Control Program. http://www.doh.wa.gov/cfh/WSC/.
Updated January 31, 2007. Accessed June 13, 2007.
- Hupke C, Camp AW, Chaufournier R, Langley GJ, Little K. Transforming diabetes health care: part 1.
Changing practice. Diabetes Spectr 2004;17:102-6.
- Centers for Disease Control and Prevention.
HIPAA privacy rule and public health. Guidance from CDC and the U.S.
Department of Health and Human Services. MMWR Morb Mortal Wkly Rep
2003;52 Suppl:1-17, 19-20.
- Improving chronic illness care. Seattle (WA): MacColl Institute for Healthcare Innovation, Group Health Center for Health Studies. http://www.improvingchroniccare.org/. Accessed June 13, 2007.
- Wagner E.
Chronic disease management:
what will it take to improve care for chronic illness? Eff Clin Pract
1998;1(1):2-4.
- Beckles GL, Williamson DF, Brown AF, Gregg EW, Karter AJ, Kim C, et al.
Agreement between self-reports and medical records was only fair in a cross-sectional study of performance of annual eye examinations among adults with diabetes in managed care. Med Care 2007;45(9):876-83.
- Gianunzio J. CDEMS user report 2007. Olympia (WA): Washington State Department of Health; 2007.
- Colias M.
Disease registries. Hosp Health Netw 2005;79(2):62-4,
66-8, 2.
Back to top
|
|
