

|

|

Volume
3:
No. 1, January 2006
ORIGINAL RESEARCH
Identifying Geographic Disparities in the Early Detection of Breast Cancer Using a Geographic Information System
Jane A. McElroy, PhD, Patrick L. Remington, MD, Ronald E. Gangnon, PhD, Luxme Hariharan, LeAnn D. Andersen, MS
Suggested citation for this article: McElroy JA, Remington PL, Gangnon RE, Hariharan L, Andersen LD. Identifying geographic disparities in the early detection of breast cancer using a geographic information system. Prev Chronic Dis [serial online] 2006 Jan [date cited]. Available from: URL: http://www.cdc.gov/pcd/issues/2006/
jan/05_0065.htm.
PEER REVIEWED
Abstract
Introduction
Identifying communities with lower rates of mammography screening is a critical step to providing targeted screening programs; however, population-based data necessary for identifying these geographic areas are limited. This study presents methods to identify geographic disparities in the early detection of breast cancer.
Methods
Data for all women residing in Dane County, Wisconsin, at the time of their
breast cancer diagnosis from 1981 through 2000 (N = 4769) were obtained from the Wisconsin Cancer Reporting System
(Wisconsin’s tumor registry) by ZIP code of residence. Hierarchical logistic regression models for disease mapping were used to identify geographic differences in the early detection of breast cancer.
Results
The percentage of breast cancer cases diagnosed in situ (excluding lobular
carcinoma in situ) increased from 1.3% in 1981 to 11.9% in 2000. This increase,
reflecting increasing mammography use, occurred sooner in Dane County than in
Wisconsin as a whole. From 1981 through 1985, the proportion of breast cancer
diagnosed in situ in Dane county was universally low (2%–3%). From 1986 through
1990, urban and suburban ZIP codes had significantly higher rates (10%) compared
with rural ZIP codes (5%). From 1991 through 1995, mammography screening had
increased in rural ZIP codes (7% of breast cancer diagnosed in situ). From 1996
through 2000, mammography use was fairly homogeneous across the entire county (13%–14%
of breast cancer
diagnosed in situ).
Conclusion
The percentage of breast cancer cases diagnosed in situ increased in the state and in all areas of Dane County from 1981 through 2000. Visual display of the geographic differences in the early detection of breast cancer demonstrates the diffusion of mammography use across the county over the 20-year period.
Back to top
Introduction
Geographic differences in health status and use of health services have been reported in the United States and internationally (1), including stage of breast cancer incidence and mammography screening practices (2). Early diagnosis of breast cancer through mammography screening improves breast cancer treatment options and may reduce mortality (3,4), yet many women in the United States are not
routinely screened according to recommended guidelines (5).
Needs assessment to account for noncompliance with breast cancer screening recommendations has focused on personal factors related to participation, including the barriers women perceive (6), the role of physicians (7), and the role of services such as mobile vans (8) and insurance coverage (9). Evaluations of the effectiveness of interventions directed at patients, communities, and special
populations have also provided important information about mammography use (10). However, little attention has been paid to geographic location, except to focus on inner-city and rural disparities in mammography use (11,12).
The purpose of this study was to identify geographic disparities in the early detection of breast cancer using cancer registry data. This information can be used to identify areas where increased mammography screening is needed and to understand the diffusion of innovation in an urban or
a rural setting.
Cancer registry data were used for these analyses. Validity of the use of
these data rests on the correlation between the percentage of breast cancer
diagnosed in situ and mammography screening rates; breast cancer in situ (BCIS)
(excluding lobular carcinoma in situ [13-15]) is the earliest stage of localized breast cancer and is diagnosed almost exclusively by mammography (16). In the 1970s, before widespread
use of mammography, BCIS represented less than 2% of breast cancer cases in the United States (15). A nationwide community-based breast cancer screening program showed that among populations of women screened regularly, the stage distribution
of diagnosed cases was skewed to earlier stages, with BCIS accounting for more than 35% (17). Trends in the relative frequency of BCIS are closely correlated with trends in
mammography use (reflected in data from surveys of mammography providers in Wisconsin) and with trends in self-reported mammography use
(reflected in data from the Behavioral Risk Factor Surveillance System) (18-20).
In Wisconsin, either a physician can refer a patient for screening or a woman
can self-refer. More than 60% of the mammography imaging facilities in the state
accept self-referrals (21). Since 1989, Wisconsin state law has mandated health
insurance coverage for women aged 45 to 65 years, and Medicare covers
mammography screening for eligible women (22). In Wisconsin, the Department of
Health and Family Services provides a toll-free number through which women can contact more than 400 service providers (22). Finally, several programs such as the Wisconsin Well Woman Program, which is funded by the Centers for Disease Control and Prevention, provide free or low-cost screening to underserved women.
Back to top
Methods
Study population
All female breast cancer cases diagnosed from 1981 through 2000 were identified by the Wisconsin Cancer Reporting System (WCRS). The WCRS was established in 1976 as mandated by Wisconsin state statute to collect cancer incidence data on Wisconsin residents. In compliance with state law, hospitals
and physicians are required to report cancer cases to the WCRS (within 6
months of initial diagnosis for hospitals and within 3 months for physicians,
through their clinics). Variables obtained from
the WCRS included histology (International Classification of Diseases for
Oncology, 2nd Edition [ICD-02] codes), stage (0 = in situ, 1 = localized, 2–5 = regional, 7 = distant, and 9 = unstaged), year of diagnosis, county of residence at time of diagnosis, and number
of incident cases in 5-year age groups by ZIP code for all breast cancer cases among women. ZIP codes and county of residences, self-reported by the women with diagnosed breast cancer, are provided to the WCRS. Only ZIP codes verified for Dane County by the U.S. Postal Service were included in the data set (n = 37). The ZIP code was the smallest area unit available for WCRS incidence data.
Study location and characteristics
Dane County is located in south central Wisconsin. The population of the county in 1990 was 367,085, with 20% of the population living in rural areas
(23); approximately 190,000 people lived in Madison, the second largest city in Wisconsin and home to the University of Wisconsin. The 37 unique ZIP codes in Dane County incorporate 60 cities, villages, and towns (Figure 1).
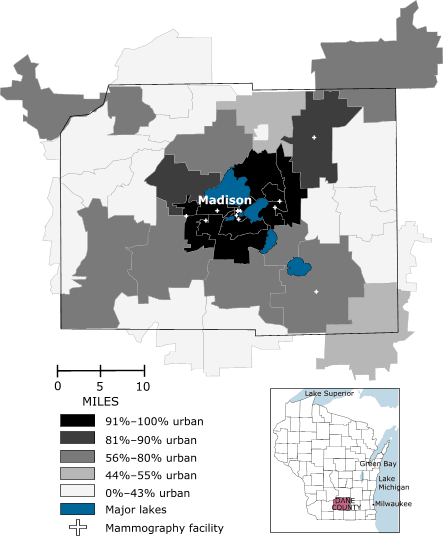
Figure 1. Map of Dane County, Wisconsin, showing capital city of Madison, major lakes, active mammogram facilities, and percentage of area classified as urban by ZIP code,
using 1996 ZIP code boundaries and 1990 census data. Inset map shows location of Dane County within
the state.
Data analysis
We determined the percentage of breast cancer cases diagnosed as BCIS in Wisconsin and Dane County over time and by ZIP codes for Dane County. For ZIP codes that
encompassed areas beyond the borders of Dane County, only women who reported their county of residence as Dane were included in the analysis. The percentage of BCIS
by ZIP code was mapped using 1996 ZIP code boundary files. For 17 breast
cancer cases in which the women’s ZIP codes no longer existed, each ZIP code was reassigned to the ZIP code in the same location.
We used analytic methods to estimate rates of early breast cancer detection
by ZIP code. Because of small numbers of BCIS cases in each ZIP code, a well-characterized statistical method was used to stabilize the prediction of rates by borrowing information from neighboring ZIP codes (24).
This is done by using Bayesian hierarchical logistic regression
models to estimate ZIP-code–specific effects on percentage of breast cancer
cases diagnosed in situ (excluding lobular carcinoma in situ). ZIP-code–specific effects (log odds ratios) were modeled as a Gaussian conditional autoregression (CAR) (25). Using the CAR model, one assumes that the log odds ratio for one ZIP code is influenced by the average log odds ratio for its neighbors. The conditional standard deviation of the CAR model, the free parameter which
controls the smoothness of the map, was given a uniform prior (24).
For each time period, two CAR models were fitted. The first model included
age group as the only covariate. Age group effects were modeled using an
exchangeable normal prior. The standard deviation of this distribution was given
a uniform prior. The second model included additional ZIP-code–level covariates.
Potential covariates were urban or rural status, education, median household
income, marital status, employment status, and commuting time from the Summary
Tape File 3 of the 1990 U.S. Census of Population and Housing (23). Census data from
1990 were used because 1990 is the midpoint of the years included in these
analyses (1981–2000). Urban or rural status was defined as percentage of women living in each of the four census classifications: urban inside urbanized area, urban
outside of urbanized area, rural farm, and rural nonfarm for each ZIP code. Education was defined as percentage of women in each ZIP code aged 25 years and older with less than a high school diploma. Median household income for each ZIP code was based on
self-reported income. Marital status was defined as women aged 25 years and older
in each ZIP code who had never been married. Employment status was defined as percentage of women aged 16 years and older in each ZIP code who worked in 1989. Full-time employment variable was defined as percentage of women 25 years and older in each ZIP code who worked at least 40 hours per week. Commuting time was divided into five categories of percentage of female workers in each ZIP code: worked at home, commuted 1
to 14 minutes,
commuted 15 to 29 minutes, commuted 30 to 44 minutes, and commuted 45 minutes or more. Age was defined as age at diagnosis. These potential covariates were initially screened using forward stepwise logistic regression models, which included ZIP code as an exchangeable (nonspatially structured) random effect. Covariates included in the best model selected using Schwarz’s Bayesian
Information Criterion (BIC) (26) were used in the second covariate-adjusted model. The covariate effects and the intercept were given posterior priors.
Posterior estimates of the age-adjusted percentage of BCIS for each ZIP code in each
time period were obtained from the CAR model. Posterior medians were used as point estimates of the parameters, and 95% posterior credible intervals were obtained. Analyses were performed using WinBUGS software (27). Covariate
screening was performed using SAS software, version 8 (SAS Institute Inc, Cary, NC). ZIP-code–specific estimates were mapped using ESRI 3.2 ArcView software (Environmental Systems Research Institute, Redwood, Calif)
and 1996 ZIP code boundary files to display the data.
As an empirical check on our mapping, we fitted a regression model to the BCIS rates by ZIP code. The dependent variable was BCIS rates (using the posterior estimates of age-adjusted percentage of BCIS), and the independent variable in the model was linear distance from the University of Wisconsin Comprehensive Cancer Center
(UWCCC), located in Madison, to the centroid of each ZIP code.
Back to top
Results
A total of 4769 breast cancer cases were reported in Dane County from 1981 through
2000: 825 from 1981 through 1985, 1119 from 1986 through 1990, 1239 from 1991
through 1995, and 1586 from 1996 through 2000. Percentage of cases in situ varied by age group from a high of 18%
among women aged 45 to 49 years to a low of 0% among women aged 20 to 24 years. From the mid 1980s, the age group most frequently
diagnosed with BCIS was women aged 45 to 49. In contrast, women aged 20 to 34 and older than 84 were the least often (≤2%) diagnosed with BCIS (data not shown). Based on the 1990 U.S.
census, the total female population (aged 18 years and older) in Dane County was 145,974; 60% of the female population had more than a high school degree, and 15% of the
female population aged 25 and older had never married.
In Dane County, the percentage of BCIS increased from 1.3% in 1981 to 11.9% in 2000. For the state, the percentage of BCIS
increased from 1.5% in 1981 to 12.8% in 2000. From 1981 to 1993, Dane County had a higher percentage of BCIS diagnosis than the state as a whole. By the mid-1990s, the percentage of BCIS among breast cancer cases in Dane County was similar to the
percentage in the state (Figure 2). Similar results are seen when mapping the observed data (maps not shown).
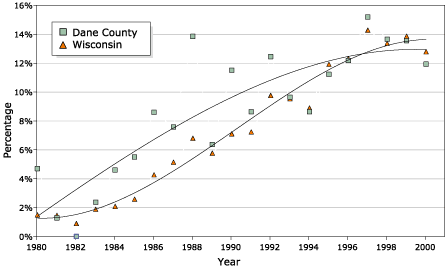
Figure 2. Smoothed trends in percentage of breast
cancer cases diagnosed in situ (excluding lobular carcinoma in situ), Dane
County, Wisconsin, and Wisconsin, 1981–2000. Data point for Dane County, 1980,
was estimated from Andersen et al (28). [A tabular
version of this graph is also available.]
Figure 3 shows model-based estimates of the age-adjusted percentage of BCIS diagnosis by ZIP code in Dane County during four 5-year periods. These maps demonstrate the increase in the percentage of cases diagnosed as BCIS noted in Figure
2. These maps also show that the increase in the percentage of BCIS was not
uniform across Dane County. From 1981 through 1985, the entire county had
uniformly low rates of BCIS (2%–3%). From 1986 through 1990, urban ZIP codes had markedly higher rates of BCIS
(approximately 12%) compared with rural ZIP codes (approximately 5%). From 1991
through 1995, use of mammography screening had begun to increase in the rural ZIP codes (with
a 7% rate of BCIS), although the rates
of BCIS remained higher in urban ZIP codes (12%). From 1996 through 2000, mammography
screening was fairly universal across the county, with BCIS rates of 13% to 14%. Similar patterns were observed from models
that adjusted for additional covariates of marital status and education (data not shown).
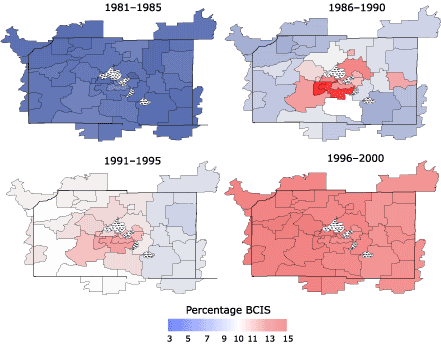
Figure 3. Model-based estimates of age-adjusted percentage of breast cancer cases diagnosed
in situ
during four 5-year periods, by ZIP code, Dane County, Wisconsin, 1981–2000. BCIS indicates breast cancer in situ.
From 1981 through 1985, there was no significant relationship between
distance from UWCCC and
the rate of BCIS (P = .27). From 1986 through 1990 and from 1991 through 1995, there
was strong evidence of an inverse relationship between distance from UWCCC and
the rate of BCIS (i.e., the closer to UWCCC, the higher the BCIS rate [P < .001] for both periods).
From 1996 through 2000, there was a nonsignificant inverse relationship
between distance from UWCCC and the rate of BCIS (P = .07).
Back to top
Discussion
The frequency of BCIS diagnosis increased substantially in Wisconsin and in
Dane County from 1981 through 2000. This increase in percentage of BCIS among diagnosed breast cancer cases is consistent with increases in self-reported mammography use, Wisconsin Medicare claims for mammography, and the number of medical imaging centers in Wisconsin (21). However, progress in mammography screening was not
uniform across Dane County, and this lack of uniformity represents a classic case of diffusion of innovation. Early adopters of mammography use lived in and near the city of Madison. We can speculate that Madison embodies one characteristic that accelerates the diffusion process: namely, a more highly educated population living in a university community with a strong medical presence. One predictor of
mammography use is education: women who are more educated are more likely to ask their physician for a referral or to self-refer (29), and the strongest predictor of mammography use is physician referral (30). Furthermore, physicians are more likely to have chosen to live in the Madison area
instead of a more rural location because they value the opportunity for regular contact with the
medical school and the medical community (31). Consequently, a greater number of interpersonal networks and
more information exchange among physicians about adoption of this innovation might have occurred earlier in the Madison medical community than in the more rural areas of the county (32).
Although median household income by ZIP code was not a predictor of mammography use in our study, the amount of disposable income by individuals, which is not captured by this variable, might also have been an important factor for early adopters (33,34). In a national study of mammography use, income was a significant predictor of repeat screening in 1987 but not in 1990 (35). In the mid-1980s, few insurance plans covered mammography screening. Therefore, women of higher socioeconomic status (SES) would have been more likely to be able to pay the cost of the mammogram. Efforts to reduce costs, such as a 1987 statewide promotional campaign sponsored by the American Cancer Society, still required a $50 copay from women who were able to self-refer for a mammogram (36).
As the use of this technology diffused outward, increasing numbers of women living in suburban and rural areas surrounding Madison elected to get a mammogram.
From 1996 through 2000, the geographic disparity in mammography use was muted, although the eastern corridor of Dane
County still had slightly lower rates of BCIS than other parts of the county. The reasons
for persistent disparity in this region of Dane County are unclear: it is unlikely to be because of proximity to mammography screening facilities, nor are the ZIP-code–level SES measures such as percentage unemployed, household income, percentage
below poverty level, or education level statistically different from the western corridor of Dane County.
Differences in the trends of early detection of breast cancer within Dane
County suggest that progress in mammography screening has not been uniform
across the county. From 1996 through 2000, while more than 14% of age-adjusted breast cancer cases were diagnosed as BCIS
in Madison, fewer than 6% of age-adjusted breast cancer cases were diagnosed as BCIS in a few outlying and more rural areas of Dane County, reflecting lower mammography
use by residents in this area. The results of an earlier analysis of these data were shared with local health department staff in rural Dane County who were working to increase early detection efforts through outreach education and referrals to providers. As suggested by Andersen et al, strategies to improve mammography use include improving access to primary care physicians, increasing the
number of mammography facilities located in rural areas, and increasing outreach efforts by a network of public health professionals promoting screening in their community (28). In addition, pointing out the variations in care may lead to improvements, since the first step toward change is identifying a problem. With identification of particular areas of need, resources can be garnered toward
alleviating the disparity.
Persistent disparities in mammography use after adjusting for community level
of educational attainment and marital status were found. Other studies have
found that patients with cancer living in census tracts with lower median levels of
education attainment are diagnosed in later disease stages than are patients in
tracts with higher median levels of education (29). Studies have also shown that
one predictor for getting a mammogram is being married (37).
This study demonstrates the use of percentage of BCIS as a tool for comparing
population-based mammography screening rates in different geographic areas.
Using cancer incidence data to monitor population-based rates of breast cancer
screening is possible throughout the nation, because data from population-based
cancer registries are now widely available, often by ZIP code or census tract.
This method permits comparison of mammography screening rates among geographic
areas smaller than areas used in many previous studies of geographic variation in the early detection of breast cancer (2).
The method described in this article can be used to complement other ways to assess the quality of health care in communities, such as the Health Plan Employer Data and Information Set (HEDIS), created by the National Committee for Quality Assurance. HEDIS addresses overall rates in managed care but does not include the underinsured or fee-for-service populations particularly at risk for inadequate screening (34).
Cancer registry data are population based; therefore, using cancer registry data is not only effective but also economical and efficient for outreach specialists and health providers.
A potential weakness in this method is the representativeness of the statewide tumor registry. However, the WCRS
has been evaluated by the North American Association of Central Cancer
Registries and was given its gold standard for quality, completeness, and
timeliness in 1995 and 1996, the first 2 years this standard was recognized (38). Completeness
estimates are a general measure of accuracy. The WCRS participated in national audits that measured completeness in 1987, 1992, and 1996 as well as one formal study in 1982. Overall, the quality of the data improved slightly
after 1994 when clinics and neighboring state data-sharing agreements were
implemented (oral communication, Laura Stephenson, WCRS, July 2005). In addition, the tumor registry has used standard methods for classifying tumor stage (e.g., in situ) throughout the entire period of the study. Incidence data from data sources of lesser quality or completeness than the WCRS would need to be carefully evaluated for use in this type of analysis.
Another limitation of this type of analysis is our use of BCIS as a proxy for mammography screening practices. Undoubtedly, some diagnoses of BCIS
result from diagnostic mammograms, but reported use of screening mammograms by individuals and medical facilities correlates strongly with percentage of BCIS over time, particularly ductal carcinoma in situ (18-20). Furthermore, we chose
to exclude lobular carcinoma in situ from our BCIS category because this
condition is often opportunistic (13-15).
A third limitation, which would be found in any type of geographic analysis, rests on the accuracy of the assignment of participants to the proper location. For area analysis (e.g., ZIP code, county), this
legitimate concern is ameliorated by using tools to check ZIP codes and county assignments for correctness. For this study, women diagnosed with breast cancer
provided their addresses, including county of residence, to their medical facilities. These addresses were forwarded to the WCRS, where quality-control checks were implemented to validate
ZIP code and county assignments. For example, reference tables of ZIP codes and
their county codes were cross-referenced to the ZIP codes and county codes of
the addresses provided by the women diagnosed with breast cancer. Inaccuracies were corrected by the WCRS (oral communication, Laura Stephenson, WCRS, January 2005).
Although there has been significant improvement in breast cancer screening
across the state and county, this study demonstrates that the improvement has
not been uniform. The maps clearly indicate for program directors and policy
makers the areas where further outreach and research should be conducted. More
specifically, this type of analysis can be used to identify specific areas (such
as ZIP codes) within a community (such as a county) with varying rates of
early-stage breast cancer. Using this method, public health professionals can
provide population-level data to all health care providers to target
interventions to improve the early detection of breast cancer in other counties
in Wisconsin and other states. Finally, this type of analysis is useful for
comprehensive cancer control efforts and can be conducted for other cancers with
effective screening methods, such as colorectal cancer.
Back to top
Acknowledgments
The authors are grateful to Dr Larry Hanrahan and Mark Bunge for advice and Laura Stephenson of the
WCRS for assistance with data.
This study was supported by National Cancer Institute grant U01CA82004.
Back to top
Author Information
Corresponding Author: Jane A. McElroy, PhD, Comprehensive Cancer Center, 610 Walnut St, 307 WARF, Madison, WI 53726. Telephone: 608-265-8780. E-mail: jamcelroy@wisc.edu.
Author Affiliations: Patrick L. Remington, MD, Comprehensive Cancer Center and Department of Population Health Sciences, University of Wisconsin, Madison, Wis; Ronald E. Gangnon, PhD, Departments of
Population Health Sciences and Biostatistics and Medical Informatics, University of Wisconsin, Madison, Wis; Luxme Hariharan,
Department of Molecular Biology, University of Wisconsin, Madison, Wis; LeAnn D. Andersen, MS, Department of Population Health Sciences,
University of Wisconsin, Madison, Wis.
Back to top
References
- Coughlin SS, Uhler RJ, Bobo JK, Caplan L.
Breast cancer screening practices among women in the United States, 2000. Cancer Causes Control 2004;15:159-70.
- Roche LM, Skinner R, Weinstein RB.
Use of a geographic information system to identify and characterize areas with high proportions of distant stage breast cancer. J Public Health Manag Pract 2002;8:26-32.
- Lee CH.
Screening mammography: proven benefit, continued controversy. Radiol Clin North Am 2002;40:395-407.
- Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE.
Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 2002;359:909-19.
- Mandelblatt J, Saha S, Teutsch S, Hoerger T, Siu AL, Atkins D, et al.
The cost-effectiveness of screening mammography beyond age 65 years: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139:835-42.
- Rimer BK. Understanding the acceptance of mammography by women. Annals of Behavioral Medicine 1992;14:197-203.
- Fox SA, Stein JA.
The effect of physician-patient communication on mammography utilization by different ethnic groups. Med Care 1991;29:1065-82.
- Haiart DC, McKenzie L, Henderson J, Pollock W, McQueen DV, Roberts MM,
et al.
Mobile breast screening: factors affecting uptake, efforts to increase response and acceptability. Public Health 1990;104:239-47.
- Thompson GB, Kessler LG, Boss LP.
Breast cancer screening legislation in the United States. Am J Public Health 1989;79:1541-3.
- Zapka JG, Harris DR, Hosmer D, Costanza ME, Mas E, Barth R.
Effect of a community health center intervention on breast cancer screening among Hispanic American women. Health Serv Res 1993;28:223-35.
- Breen N, Figueroa JB.
Stage of breast and cervical cancer diagnosis in disadvantaged neighborhoods: a prevention policy perspective.
Am J Prev Med 1996;12:319-26.
- Andersen MR, Yasui Y, Meischke H, Kuniyuki A, Etzioni R, Urban N.
The effectiveness of mammography promotion by volunteers in rural communities. Am J Prev Med 2000;18:199-207.
- Li CI, Anderson BO, Daling JR, Moe RE.
Changing incidence of lobular carcinoma in situ of the breast. Breast Cancer Res Treat 2002;75:259-68.
- Millikan R, Dressler L, Geradts J, Graham M.
The need for epidemiologic studies of in-situ carcinoma of the breast. Breast
Cancer Res Treat 1995;35:65-77.
- Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C.
Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA 1996;275:913-8.
- Claus EB, Stowe M, Carter D.
Breast carcinoma in situ: risk factors and screening patterns. J Natl Cancer Inst 2001;93:1811-7.
- May DS, Lee NC, Richardson LC, Giustozzi AG, Bobo JK.
Mammography and breast cancer detection by race and Hispanic ethnicity: results from a national program (United States). Cancer Causes Control 2000;11:697-705.
- Lantz P, Bunge M, Remington PL.
Trends in mammography in Wisconsin. Wis Med J 1990;89:281-2.
- Lantz PM, Remington PL, Newcomb PA.
Mammography
screening and increased incidence of breast cancer in Wisconsin. J Natl Cancer Inst 1991;83:1540-6.
- Bush DS, Remington PL, Reeves M, Phillips JL.
In situ breast cancer correlates with mammography use, Wisconsin: 1980-1992. Wis Med J 1994;93:483-4.
- Propeck PA, Scanlan KA.
Breast imaging trends in Wisconsin.
WMJ 2000;99:42-6.
- Fowler BA.
Variability in mammography screening legislation across the states. J Womens Health Gend Based Med 2000;9:175-84.
- U.S. Census Bureau. 1990 Summary Tape File 3 (STF 3)- sample data. Census of Population and Housing.
Washington (DC): American Factfinder;1990. Available from: URL: http://www.census.gov/main/www/cen1990.html*.
- Gelman A. Prior distributions for variance parameters in hierarchical
models. New York: Columbia University; 2004.
- Besag J, York J, Mollié A. Bayesian image restoration with applications in spatial statistics. Annals of the Institute of Mathematical Statistics 1991;43:1-20.
- Schwarz G. Estimating the dimension of a model. Annals of Statistics 1978;6:461-4.
- Spiegelhalter D, Thomas A, Best N, Lunn D. WinBUGS: Bayesian inference using Gibbs Sampling.
London (UK): MRC Biostatistics Unit; 2004.
- Andersen LD, Remington PL, Trentham-Dietz A, Robert S.
Community trends in the early detection of breast cancer in Wisconsin, 1980-1998. Am J Prev Med 2004;26:51-5.
- Wells BL, Horm JW.
Stage at diagnosis in breast cancer: race and socioeconomic factors. Am J Public Health 1992;82:1383-5.
- Simon MS, Gimotty PA, Coombs J, McBride S, Moncrease A, Burack RC.
Factors affecting participation in a mammography screening program among members of an urban Detroit health maintenance organization.
Cancer Detect Prev 1998;22:30-8.
- Cooper JK, Heald K, Samuels M, Coleman S.
Rural or urban practice: factors influencing the location decision of primary care physicians. Inquiry 1975;12:18-25.
- Rogers EM. Diffusion of innovations. New York (NY): Free Press; 2003.
- Calle EE, Flanders WD, Thun MJ, Martin LM.
Demographic predictors of mammography and Pap smear screening in US women. Am J Public Health 1993;83:53-60.
- Bradley CJ, Given CW, Roberts C.
Disparities in cancer diagnosis and survival. Cancer 2001;91:178-88.
- Zapka JG, Hosmer D, Costanza ME, Harris DR, Stoddard A.
Changes in mammography use: economic, need, and service factors. Am J Public Health 1992;82:1345-51.
- Remington PL, Lantz PM.
Using a population-based cancer reporting system to evaluate a breast cancer detection and awareness program. CA Cancer J Clin 1992;42:367-71.
- Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS.
Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA 1998;279:1801-7.
- Chen VW, Wu XC, Andrews PA. Cancer in North America, 1991-1995, Vol I: Incidence. Sacramento (CA): North American Association of Central Cancer Registries; 1999.
Back to top
*URLs for nonfederal organizations are provided solely as a
service to our users. URLs do not constitute an endorsement of any organization
by CDC or the federal government, and none should be inferred. CDC is
not responsible for the content of Web pages found at these URLs.
|
|
