 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Risk Factors for Neonatal Tetanus --- Busoga Region, Uganda, 2002--2003Sheba N. Gitta,1 F.
Wabwire-Mangen,1 D.
Kitimbo,2 G. Pariyo1
Corresponding author: Sheba N. Gitta, Makerere University Institute of Public Health, P.O. Box 7072, Kampala, Uganda. Telephone: 256-77-2479403; Fax: 256-41-531807; E-mail: sgitta@iph.ac.ug or sgitta@med.mak.ac.ug. Disclosure of relationship: The contributors of this report have disclosed that they have no financial interest, relationship, affiliation, or other association with any organization that might represent a conflict of interest. In addition, this report does not contain any discussion of unlabeled use of commercial products or products for investigational use. AbstractBackground: Uganda has not achieved the 2005 neonatal tetanus (NNT) global elimination target set by the World Health Organization (WHO). The Busoga region has the highest recorded level of NNT incidence in Uganda. To understand the reasons for this high incidence, a study was conducted to identify NNT risk factors. Methods: During March--May 2004, a matched case-control study was conducted in the Busoga region for a 2-year study period (2002--2003). Matching variables were sex, residence, and date of birth. A total of 24 cases of NNT (according to the WHO case definition) were identified from hospital records, and 96 community controls (children who survived the neonatal period) were selected. Results: Bivariate analysis indicated that neonates with NNT were more likely to have been delivered outside a health facility, on an unclean surface, without use of gloves, or by unskilled attendants. Mothers of these neonates were less likely to report vaccination during previous pregnancies, administration of 2 doses of tetanus toxoid (TT) during the study pregnancy, or use of certain intravaginal substances (most commonly, herbs) at onset of labor. Multivariate analysis indicated that unclean delivery surfaces (odds ratio [OR] = 38.8; 95% confidence interval [CI] = 2.9--518.1) and primigravidae mothers (OR = 79.5; CI = 1.8--3,472.2) were associated with NNT. Administration of 2 doses of TT during pregnancy, vaccination during previous pregnancies, and intravaginal application of certain substances were protective against NNT. Conclusion: These findings underscore the importance of having clean delivery surfaces and of mothers receiving 2 doses of TT during pregnancy. Implementation of these measures might help eliminate NNT from the Busoga region of Uganda. IntroductionNeonatal tetanus (NNT) is a fatal yet preventable disease that accounts for 14% of annual neonatal deaths worldwide (1). In 1997, a total of 248,000 NNT deaths occurred globally; 95,000 NNT deaths occurred in Africa, 4,600 of which occurred in Uganda (2). In 2000, the World Health Organization (WHO) targeted NNT for global elimination by 2005 (3). Uganda is one of the 57 countries that have not yet eliminated NNT. In Uganda, with 195 cases, the Busoga region has the highest NNT incidence (4). In 2002, the Uganda Ministry of Health (MoH) launched an NNT elimination campaign in Busoga. After the second round of mass tetanus toxoid (TT) immunization during the NNT elimination campaign, approximately 86% of women of reproductive age in all districts in the region had received 2 doses of TT (5). A previous study attributed NNT in Busoga to unhygienic delivery practices, application of harmful substances on the umbilical cord, and lack of TT immunization among mothers but did not document the magnitude of risk associated with these factors (6). Worldwide, NNT has been associated with prenatal, perinatal, and neonatal factors, including unhygienic delivery and cord-care practices and lack of maternal TT immunization (7). Risk for contamination and subsequent occurrence of NNT remains high for several days after delivery until the baby's cord wound heals (8). NNT cases continue to be reported in Busoga. For this reason, district health teams in the region recommended that a study be conducted to identify NNT risk factors as a basis for planning and implementing appropriate NNT elimination strategies. MethodsA community-based, matched case-control study was conducted to identify delivery and umbilical cord-care practices that predispose neonates to NNT and to compare maternal TT immunization status for both groups. The study was conducted during March--May 2004 in five districts that make up the Busoga region (Bugiri, Iganga, Jinja, Kamuli, and Mayuge). Respondents were the mothers of the neonates with NNT and matched controls. Mothers of neonates with NNT were identified by screening NNT investigation forms from all hospitals in the Busoga region for January 2002--December 2003. A case was defined as confirmed NNT according to the WHO case definition (i.e., history of normal sucking and crying for the first 2 days of life, onset of illness at age 3--28 days, and inability to suck followed by generalized stiffness and/or spasms) occurring in a child born in the Busoga region during the study period (5). A control was defined as a child who had survived the neonatal period, who was matched for sex and date of birth (range: ±6 months), and who lived in the same village as a neonate with NNT. A control was selected from each of the first four households closest to the home of a child with NNT that had a child consistent with the control definition. If a household had more than one eligible control, the child whose date of birth was closest to that of the neonate with NNT was selected. Multiple controls were selected to increase the power of the study (9). Children whose mothers did not live in the Busoga region at the time of delivery were excluded from the study. The questionnaire used was adapted from the WHO NNT case investigation form (5). It was modified before the study was conducted on the basis of qualitative data about the most probable NNT risk factors collected from 80 mothers who participated in eight focus group discussions and interviews with eight traditional birth attendants. Questionnaires were pretested among 20 Jinja district mothers who were not part of the final study sample. Trained interviewers used the pretested, interviewer-administered questionnaire to collect data on maternal sociodemographic characteristics, delivery practices, umbilical cord-care practices, and maternal TT immunization status. The delivery surface was categorized as clean or unclean; any surface other than a new plastic sheet or operating theater table was classified as unclean. The mother of a neonate with NNT was always interviewed before that of the controls to ensure accurate matching. The Uganda National Council of Science and Technology and Makerere University Institute of Public Health Higher Degrees Research and Ethics Committee approved this study, and all participating mothers signed consent forms. Data were double-entered and validated by using Epi Info 6.04 (CDC, Atlanta, Georgia). Epi Info also was used for bivariate analysis; stratified Mantel-Haenszel analysis produced matched odds ratios (ORs) and 95% confidence intervals (CIs). For multivariate analysis, data were exported to SPSS 10.0 (Statistical Package for the Social Sciences for Windows, version 10.0; SPSS; Chicago, Illinois); conditional logistic regression was performed to identify the predictors for NNT. All variables entered in the model were dichotomized, with neonatal tetanus case (Yes/No) as the outcome variable. The initial logistic regression model included all variables that were statistically significant during bivariate analysis (e.g., delivery place, birth attendant, delivery surface, use of intravaginal substances, use of gloves, maternal TT status in study, and previous pregnancies). Other variables that were not significant on bivariate analysis but that are known risk factors for NNT (e.g., handwashing, type of cord-cutting tool, cord dressings, and cord tie used) and potential confounders (e.g., maternal age) also were included in the model. Measures of interest were adjusted ORs and CIs for NNT risk factors. Goodness of fit of the final model was tested by using Nagelkerke R2 value. ResultsA total of 40 NNT cases were identified from the records, but only 24 mothers could be traced in the community. Full names were not available for three mothers, and addresses of nine mothers were not recorded in the hospital records. In four cases, parents had moved and could not be located. Four matched controls were selected for each of the 24 cases, for a total of 96 controls and a study sample of 120. Bivariate AnalysisThe majority of neonates with NNT and controls had mothers who were aged >20 years, were currently married, and had attained at least a primary education (Table 1). The percentage of mothers who were primigravidae (i.e., this was their first pregnancy) was 25% for neonates with NNT and 12.5% for controls. The majority of mothers (17 [70.8%] of 24 neonates with NNT and 62 [64.6%] of 96 controls) had had one to four previous deliveries. No statistically significant associations were observed between maternal sociodemographic characteristics and NNT. Maternal TT immunization status of the two study groups was compared (Table 2). Maternal TT immunization status was determined by history because only 12.5% of mothers whose neonates had NNT and 26% of controls had TT immunization cards. Significantly fewer mothers of neonates with NNT (20.8%) than control mothers (66.7%) had received the recommended 2 doses of TT by the time of delivery (OR = 0.2; CI = 0.1--0.5; p = 0.0001). Mothers of neonates with NNT also were less likely to have had TT immunization for previous births (OR = 0.1; CI = 0.02--0.3; p<0.001). The majority of mothers in both groups had received prenatal care during the study pregnancy, but this was not identified as being protective. All four investigated delivery practices were associated with NNT (Table 2). Mothers of neonates with NNT were more likely than control mothers to have delivered outside health facilities and to have had unskilled birth attendants; 70.8% of these mothers had unskilled attendants, including traditional birth attendants (six of 24), friends or relatives (six of 24), and nursing assistants (five of 24). In contrast, only 31.3% of controls were delivered by unskilled attendants, including traditional birth attendants (12 of 96), friends or relatives (nine of 96), nursing assistants (four of 96), and self (five of 96). Doctors delivered no neonates with NNT and 3.1% (three of 96) of controls. The rest were delivered by midwives and nurses. Mothers of neonates with NNT were more likely than control mothers to have delivered on unclean delivery surfaces (e.g., mats, sacks, uncovered delivery beds, and uncovered floors). Certain mothers (three [12.5%] of 24 mothers of children with NNT and 36 [37.5%] of 96 controls) reported having applied substances into the vagina at onset of labor, a traditional practice in this region; mothers of neonates with NNT were less likely than control mothers to have used intravaginal substances (most frequently herbs) (OR = 0.2; CI = 0.1--0.8) (Table 3). Bivariate analysis indicated that the only umbilical cord-care practice that was associated with NNT was the birth attendant not wearing gloves (OR = 3.8; CI = 1.1--13.1; p = 0.06) (Table 2). Multivariate AnalysisThe best fitting model for NNT risk factors explained 71.4% (Nagelkerke R2) of the variation observed in the outcome variable. Within this model, the mother receiving 2 doses of TT during the study pregnancy and a history of receiving TT in previous pregnancies were significantly protective (p = 0.007) (Table 4). Receiving only 1 TT dose in study pregnancy was not protective and was excluded from the final model. Delivery on an unclean surface and primigravidae mothers were associated with increased risk for NNT. Use of intravaginal substances at onset of labor was significantly protective, with mothers of neonates with NNT less likely to have used them (p = 0.017). DiscussionThis study identified two risk factors for NNT and two protective factors in the Busoga region of Uganda: unclean delivery surfaces and primigravidae mothers. Unclean delivery surfaces were the most likely source of tetanus organisms, underscoring the importance of using clean delivery surfaces to prevent NNT. This finding concurs with results of other studies that identified unclean delivery surfaces as a risk factor for NNT (8,10). Children born to primigravidae mothers were at more risk for having NNT than those born to multiparous mothers. The association might be confounded by receipt of TT before pregnancy, which was not captured in this study. Intravaginal application of local medicines (most commonly, herbs) at onset of labor is widely practiced as it is thought to ease labor by widening the birth canal. This traditional practice was negatively associated with acquisition of NNT, implying that it might have had a protective effect. This finding has not been reported previously and requires further clarification. Association might result from reporting bias, as a mother of a neonate with NNT conceivably might have withheld information about use of these substances for fear of being blamed for her child's illness. Alternatively, applying such substances immediately after bathing, as is the practice, might have ensured that clean hands were used, thus decreasing the risk for contaminating the substances and the birth canal. This finding is contrary to the increased risk for NNT observed in other studies of pregnant women who had predelivery intravaginal exposure to ghee or coconut oil (8,11). Possibly the herbs used as intravaginal medications had antibacterial properties. Further research is required to determine if these herbs have such medicinal properties. Absence of the mother receiving 2 doses of TT vaccination during pregnancy was identified as a risk factor for NNT, which concurs with findings of previous studies conducted in Uganda and Nigeria (6,11,12). The study indicated that <80% of all mothers in Busoga region had received recommended 2 doses of TT, which suggests that >20% of children born in the region are still at risk for having NNT because of low 2-dose TT coverage. A discrepancy was noted between prenatal care attendance and maternal TT vaccination. Although approximately 75% of mothers of neonates with NNT had prenatal care, only half received >1 dose of TT, indicating that health-care workers are missing opportunities to vaccinate pregnant women. This finding has been documented previously (13--15). This discrepancy might result from mothers receiving prenatal care only when pregnancy is so far advanced that they can receive only 1 dose of TT before delivery. The type of tool used to cut the umbilical cord was not identified as a risk factor for NNT. This finding is consistent with a previous study conducted in Senegal (16) and is probably attributable to the use of new razor blades and scissors. In addition, no mother reported having applied any harmful substance (e.g., cow dung or mud) to the newborn's cord wound. This suggests that a positive shift in cord-care culture has occurred among mothers in Busoga; certain unhygienic cord practices identified previously are no longer practiced (6). This change in cord-care practices might be attributable to widespread health education activities in this region, especially during recent mass NNT elimination campaigns. It also might result from public awareness of risk for acquisition of HIV associated with use of unsterilized instruments. The findings in this report are subject to at least five limitations. First, use of unverified maternal TT vaccination histories might have biased study findings. Second, health education provided during the NNT elimination campaign might have introduced reporting bias; however, such bias would be nondifferential. Third, recall bias might have occurred because mothers of neonates with NNT are likely to have a better recall of circumstances surrounding delivery and cord care than control mothers. Birth-date matching attempted to equalize recall difficulties for both mothers of cases and controls. Fourth, NNT status of participants could not be masked; however, nonmedical data collectors were employed to minimize interviewer bias. Finally, only 60% of mothers of neonates with NNT could be traced, which reduced study sample size. However, the matched case-control study design with four controls per case minimized the effect of this limitation. ConclusionThis study identified two risk factors for NNT: unclean delivery surfaces and primigravidae mothers. Protective factors were the mother receiving 2 doses of TT in current pregnancy, history of TT in a previous pregnancy, and application of intravaginal substances at onset of labor. Cord-care practices were not NNT risk factors. On the basis of these findings, the following recommendations are offered:
References
Table 1 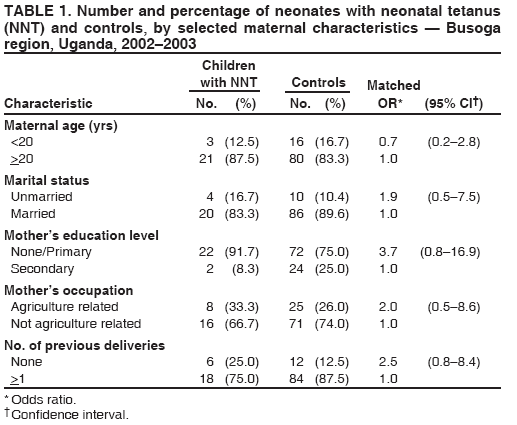 Return to top. Table 2 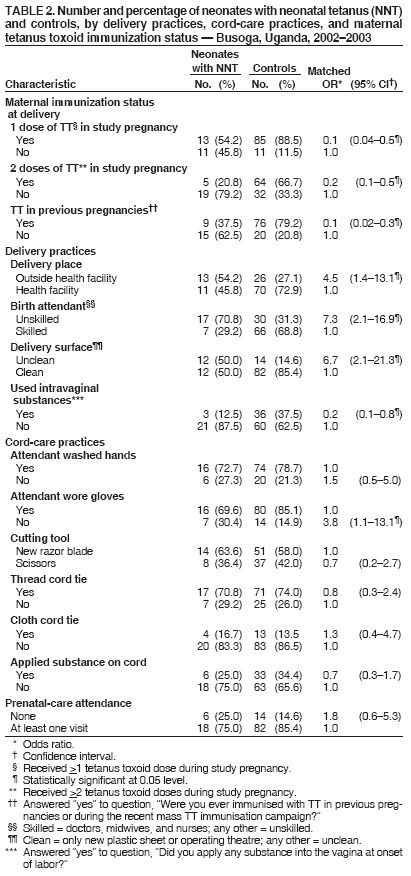 Return to top. Table 3 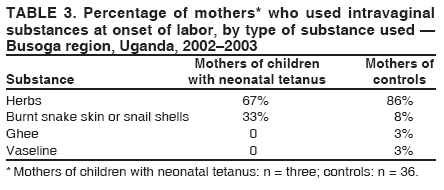 Return to top. Table 4 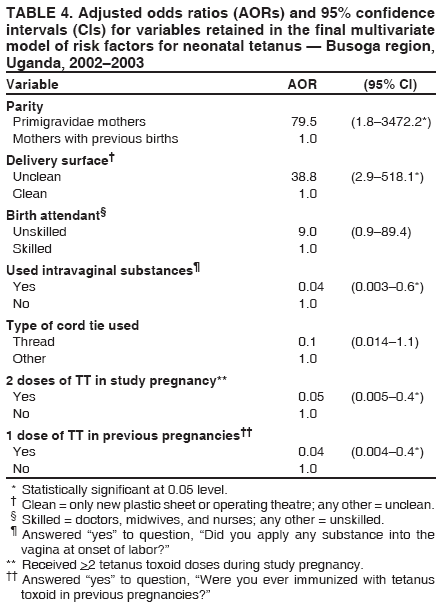 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 4/6/2006 |
|||||||||
|