 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Bacterial Meningitis Among Cochlear Implant Recipients --- Canada, 2002Samantha D. Wilson-Clark,1,2 S.
Squires,1 S. Deeks1
Corresponding author: Samantha D. Wilson-Clark, Region of Waterloo Public Health, 99 Regina St S, 3rd floor, Waterloo, ON N2J 4V3. Telephone: 519-883-2004 ext. 5413; Fax: 519-883-2241; E-mail: wsamanth@region.waterloo.on.ca. Disclosure of relationship: The contributors of this report have disclosed that they have no financial interest, relationship, affiliation, or other association with any organization that might represent a conflict of interest. In addition, this report does not contain any discussion of unlabeled use of commercial products or products for investigational use. AbstractIntroduction: In July 2002, a cluster of bacterial meningitis (BM) cases was identified among European cochlear implant recipients (CIRs), prompting Health Canada to conduct a retrospective cohort study to determine the rate of BM infection among Canadian CIRs and to identify risk factors for acquiring BM. Methods: A survey was mailed to 1,432 Canadian CIRs who had received implants during January 1995--July 2002 to assess occurrence of postimplant BM infection. Data collection included demographics, episodes of meningitis, and vaccination status. Results: A total of 1,024 (72%) surveys were completed. Median age of CIRs at implantation was 16 years (range: 7 months--81 years). Five (0.5%) cases of BM infection were reported (two pneumococcal, one meningococcal, and two of unknown etiology); one CIR died. Four cases occurred among children aged <18 years. Time between implantation and BM infection varied (range: 7 months--7.7 years; median: 11 months). The rate of BM infection per 1,000 person-years was 0.7 among CIRs aged >18 years and 2.9 among those aged <18 years. The proportion of CIRs vaccinated against pneumococcal and meningococcal disease was low (46% and 41%, respectively). Preimplant meningitis was identified as a risk factor for postimplant BM (p = 0.002). No other risk factors evaluated were associated with an increased risk for BM infection. Conclusion: CIRs have a high rate of postimplant BM infection. Preimplant BM infection was identified as a risk factor. Cases of BM infection might have been prevented through vaccination. IntroductionMeningitis is an inflammation of the lining of the brain's surface, often as a result of a bacterial or viral infection. Children aged <2 years are most at risk for meningitis. Among infants, early symptoms of meningitis include fever, irritability, lethargy, and loss of appetite. Among children and adults, other symptoms might include headache, stiff neck, photophobia, nausea and vomiting, and confusion or alteration in consciousness (1). Cochelar implants are medical devices that electronically stimulate the auditory nerves in the cochlea (inner ear), allowing persons with severe hearing loss to perceive sound. In early July 2002, a cluster of cases of bacterial meningitis (BM) infection was identified among European cochlear implant recipients (CIRs) who had received an implant produced by manufacturer A (2). European implant surgeons had hypothesized that the cluster of BM infections they had observed among their patients might be related to a recently licensed cochlear implant device (device A) produced by manufacturer A (2). This device differed from other similar manufactured devices in that it had two components, an electrode array and a positioner, rather than a single electrode array. The positioner increased electrical signal transmission to the auditory nerve, particularly among patients with malformations of the cochlea. On July 23, manufacturer A issued a voluntary recall of the device in France (3). After discussions with the European regulatory authorities, manufacturer A subsequently issued a voluntary worldwide withdrawal of the device. On July 26, the two-piece device, which had been licensed for use in Canada in November 1998, was withdrawn from the Canadian market. On July 29, Health Canada issued an alert warning that CIRs might be at greater risk for meningitis (4). Subsequently, the Immunization and Respiratory Infections Division of Health Canada (now the Public Health Agency of Canada) investigated the extent of BM infection among CIRs in Canada. To understand the magnitude of this problem in Canada, a retrospective cohort study was conducted. The threefold purpose of the investigation was to determine the rate of BM among CIRs in Canada, identify risk factors for postimplant BM, and recommend public health action based on investigation findings. MethodsThe study cohort included all 1,432 recipients of cochlear implant devices in Canada with implant dates during January 1995--July 2002. Cohort members were identified using manufacturer implant registries. At the time of the study, two manufacturers were licensed in Canada. A case was defined as one occurring in a CIR who reported having BM infection since receiving an implant. A self-administered questionnaire in both French and English was mailed to CIRs or, if deceased, their next of kin. The questionnaire was designed by modifying tools developed for a similar investigation in the United States (5). The survey was completed by the recipient or by a parent or guardian if the recipient was aged <16 years or was incapable of completing the survey. Questions addressed included cause of hearing loss, history of meningitis infection, vaccination status, and risk factors (e.g., household smoking, other children in the household, and otitis media infections). Questions were divided into two periods: before and after receiving a cochlear implant. Only nonnominal data were collected. Data were collected during November 25, 2002--March 31, 2003. To obtain the highest response rate possible to the mailed questionnaire, Dillman's Total Design Method for mailed surveys was followed, with certain modifications (6). Ethics approval for the study was obtained from Health Canada's Research Ethics Board. Data were entered in EpiData 2.1a (The EpiData Association, Odense, Denmark, 2001--2002). Univariate and bivariate analysis, including relative risks and chi-square tests, were conducted using Epi Info 6.04d (CDC, Atlanta, Georgia). To determine if the introduction of the two-piece implant affected incidence of BM infection, data were stratified by implant manufacturer and date of implant (i.e., before and after January 1, 1999, when the two-piece implant device was licensed in Canada). ResultsA total of 1,024 CIRs completed and returned the survey (overall response rate: 72%). Median follow-up time (from implant to BM infection or end of the study period) for CIRs was 42 months (Table 1). Among respondents, males and females were equally represented, and approximately half (n = 482) were aged <18 years. Manufacturers were represented in proportion to their market share (approximately 33% for manufacturer A and 67% for a second manufacturer [manufacturer B]). Among 98 CIRs who had an episode of meningitis infection before receiving a cochlear implant, 71 (72%) had infections that were bacterial. Of seven cases of meningitis reported after a cochlear implant, five (71%) were bacterial; the type of bacteria was reported for three cases. Two CIRs reported Streptococcus pneumoniae as the causative agent, and one reported Neisseria meningitidis (Table 2). None of the BM episodes occurred during the perioperative period (0--30 days postimplantation). The overall incidence of BM infection for this cohort was 1.8 per 1,000 person-years of observation (95% confidence interval [CI] = 0.6--4.2). Among CIRs aged <18 years, incidence was 2.9 (CI = 0.8--7.3); among CIRs aged <6 years, incidence was slightly lower (2.0; CI = 0.2--7.1). Incidence among adults aged >18 years was 0.7 per 1,000 person-years of observation (CI = 0.0--4.1). Incidence of BM infection among adults aged >18 years before and after 1999 did not vary (2.2 and 2.0 per 1,000 person-years observation, respectively). Although not statistically significant, incidence of BM infection among persons aged <18 years was higher on or after January 1, 1999, than before (4.0 and 2.2 per 1,000 person-years, respectively). Among children aged <6 years, incidence was 1.5 (CI = 0.0--8.1) before 1999 and 3.1 on or after January 1, 1999 (CI = 0.1--17.2). All five persons with BM infection had received meningococcal vaccine, and four had received pneumococcal vaccine. However, of the three persons for whom the causative agent was known, none had received vaccination against the implicated agent before the postimplant episode of meningitis. Potential risk factors assessed for postimplant BM infection included a history of otitis media, household smoking, and children living in the household. None was statistically significant (Table 3). A previous episode of BM infection was identified as a risk factor for postimplant BM infection (relative risk [RR] = 23.1; CI = 3.2--197.3; p = 0.002). Having had other implanted devices was associated with an increased risk for BM infection; however, this association was not statistically significant (p = 0.081). No difference in risk for BM infection by implant manufacturer was noted (p = 1.0). The cohort included recipients who had received the device with a positioner; however, the type of implanted device (with or without a positioner) was not well reported, and whether the positioner was an independent risk factor could not be determined. DiscussionThe rate of BM infection per 1,000 person-years among CIRs aged >18 years was 0.7, compared with 2.9 among CIRs aged <18 years. Results of a similar study of CIRs in the United States during the same period have been published (5). Incidence of BM infection among CIRs was 3.9 among U.S. children aged <6 years, compared with 2.0 among Canadian children in the same age group during the period when device A was on the market. Although different methodologies were used, certain key study questions were similar, allowing comparisons between the two studies. In Canada, no cases of perioperative BM infection were identified among CIRs, whereas in the U.S. study, the rate of perioperative BM infection was 2.1 cases per 1,000 procedures (5). Why incidence of perioperative infections is higher among CIRs in the United States is not known. These findings of increased incidence of BM infection among CIRs aged <18 years since the device with the positioner was introduced in 1998 are similar to findings published previously (5). No increased risk for BM infection was identified among adult CIRs after the device with the positioner was introduced. In Canada, surveillance of invasive diseases (e.g., meningitis) is organism specific and includes all forms of invasive disease. Incidence of invasive pneumococcal disease ranges from 11.6 to 17.3 per 100,000 population, whereas incidence of invasive meningococcal disease ranges from 0.6 to 1.6 per 100,000 population (7,8), compared with an observed incidence of 1.8 per 1,000 person-years among CIRs in this study. CIRs have multiple potential underlying conditions that might increase their risk for BM infection above that of the general Canadian population. The ideal comparison group for CIRs would be a population of severe-to-profoundly deaf persons who do not have cochlear implants; however, such data are not available. During 1994--2001, the overall annual incidence of BM infection in the general Canadian population ranged from 3.2 to 3.7 per 100,000 population (9). Preliminary study results were presented to Canada's National Advisory Committee on Immunization (NACI). In February 2003, NACI recommended that CIRs be considered at high risk for both Haemophilus influenzae type b (Hib) and invasive pneumococcal disease and should receive vaccination according to the high-risk schedule (10). In addition, CIRs, like all Canadians, should be up-to-date on all routine vaccinations, including meningococcal C conjugate vaccines. These vaccines are recommended for all Canadian children aged <5 years, adolescents, and young adults. CIRs, their parents, and caretakers should be aware of the signs and symptoms of meningitis and seek medical attention if they occur. Medical professionals should be aware of the potential for BM infection among CIRs, be vigilant for the signs and symptoms of meningitis in this population, and educate their patients accordingly. Primary health-care providers of CIRs or persons considering cochlear implants should ensure that these persons are fully vaccinated according to NACI guidelines (10). The findings in this report are subject to at least four limitations. First, the retrospective cohort was established by using manufacturer registry data that were likely incomplete and that did not contain the most recent mailing address of every CIR in Canada. CIRs who did not register their implant devices were not included in the cohort. However, because incomplete mailing addresses were evenly distributed among the two implant manufacturers, no selection bias was likely introduced as a result. Second, as with all self-administered questionnaires, reported medical histories might not be accurate, particularly for adults who might have lost their hearing many years before receiving a cochlear implant. Third, medical details (e.g., type of meningitis and whether treatment was received for episodes of otitis media) were poorly reported. Response rates for medical details varied widely (range: 40%--80%) compared with responses to other questions (e.g., ever having otitis media, ever having meningitis, and cause of deafness [range: 90%--99%]). The cause of BM was based on self-report and was not verified with medical records. In addition, subtyping of bacteria that caused postimplant BM was not reported. The type might not have been vaccine preventable. Certain questions pertaining to vaccination history were misinterpreted. For example, Hib vaccine was often indicated as having been received yearly, indicating confusion between Hib vaccine and annual influenza vaccine. Finally, the study was conducted in English and French. CIRs who could not read either of these languages were systematically excluded from the cohort and might have BM at a different rate from CIRs who read English or French. ConclusionIn this study, CIRs had postimplant BM infection at a rate of approximately 1.8 cases per 1,000 person-years of observation. In addition, children with cochlear implants had BM infection at a higher rate than adults (2.9 and 0.7 per 1,000 person-years of observation, respectively). Because of the increased risk for BM infection among CIRs, health-care professionals should ensure that CIRs and persons considering cochlear implants are vaccinated against bacteria that commonly cause meningitis. The only risk factor identified for having postimplant BM was preimplant BM infection. CIRs and their families should be aware of the signs and symptoms of meningitis and seek prompt attention if they occur. Acknowledgments Six other members of the Cochlear Implants and Bacterial Meningitis Collaborative Working Group contributed to this report: Attar Chawla, MD, Barbara Harrison, Irwin Hinberg, MD, Damian Kakwaya, Fred Lapner, MD, and Tara Tucker, MD. Lee Lior, MD, Eleni Galanis, MD, and Arlene King, MD, also contributed to this report. References
Table 1 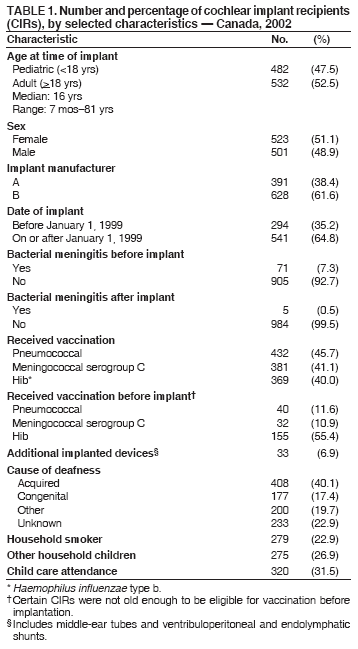 Return to top. Table 2 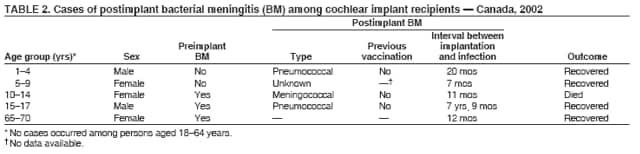 Return to top. Table 3 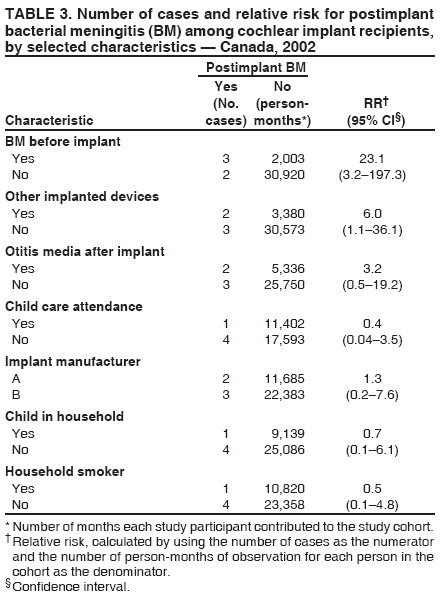 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 4/6/2006 |
|||||||||
|