 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Malaria Surveillance --- United States, 2006Please note: An erratum has been published for this article. To view the erratum, please click here. Sonja Mali, MPH
Corresponding author: Sonja Mali, MPH, Division of Parasitic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, CDC, 4770 Buford Hwy., N.E., MS F-22, Atlanta, GA 30341. Telephone: 770-488-7757; Fax: 770-488-4465; E-mail: smali@cdc.gov.
AbstractProblem/Condition: Malaria in humans is caused by intraerythrocytic protozoa of the genus Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, and P. malariae). These parasites are transmitted by the bite of an infective female Anopheles species mosquito. The majority of malaria infections in the United States occur among persons who have traveled to areas with ongoing malaria transmission. In the United States, cases can occur through exposure to infected blood products, congenital transmission, or local mosquitoborne transmission. Malaria surveillance is conducted to identify episodes of local transmission and to guide prevention recommendations for travelers. Period Covered: This report summarizes cases in persons with onset of illness in 2006 and summarizes trends during previous years. Description of System: Malaria cases confirmed by blood film or polymerase chain reaction (PCR) are mandated to be reported to local and state health departments by health-care providers or laboratory staff members. Case investigations are conducted by local and state health departments, and reports are transmitted to CDC through the National Malaria Surveillance System (NMSS), National Notifiable Diseases Surveillance System (NNDSS), and direct CDC consultations. Data from these reporting systems serve as the basis for this report. Results: CDC received reports of 1,564 cases of malaria among persons in the United States with onset of symptoms in 2006, six of which were fatal. This is an increase of 2.4% from the 1,528 cases reported for 2005. P. falciparum, P. vivax, P. malariae, and P. ovale were identified in 39.2%, 17.6%, 2.9%, and 3.0% of cases, respectively. Ten patients (0.6%) were infected by two or more species. The infecting species was unreported or undetermined in 36.6% of cases. Compared with 2005, the largest increases in cases were from Asia (16.0%). Based on estimated volume of travel, the highest estimated relative case rates of malaria among travelers occurred among those returning from West Africa. Of 602 U.S. civilians who acquired malaria abroad and for whom chemoprophylaxis information was known, 405 (67.3%) reported that they had not followed a chemoprophylactic drug regimen recommended by CDC for the area to which they had traveled. Seventeen cases were reported in pregnant women, among whom only one reported taking chemoprophylaxis precautions. Six deaths were reported; five of the persons were infected with P. falciparum and one with P. malariae. Interpretation: Despite the 2.4% increase in cases from 2005 to 2006, the numbers of malaria cases remained relatively stable during 2001--2006. No change was detected in the proportion of cases by species responsible for infection. U.S. civilians traveling to West Africa had the highest estimated relative case rates. In the majority of reported cases, U.S. civilians who acquired infection abroad had not adhered to a chemoprophylaxis regimen that was appropriate for the country in which they acquired malaria. Public Health Actions: Additional investigations were conducted of the six fatal cases that occurred in the United States. Persons traveling to a malarious area should take one of the recommended chemoprophylaxis regimens appropriate for the region of travel and use personal protection measures to prevent mosquito bites. Any person who has been to a malarious area and who subsequently has a fever or influenza-like symptoms should seek medical care immediately and report their travel history to the clinician; investigation should always include blood-film tests for malaria, with results made available immediately. Malaria infections can be fatal if not diagnosed and treated promptly. CDC recommendations concerning malaria prevention are available at http://wwwn.cdc.gov/travel/contentdiseases.aspx#malaria or by calling the CDC Malaria Branch on weekdays (telephone: 770-488-7788; Monday--Friday, 8:00 A.M.--4:30 P.M. EST); during evenings, weekends, and holidays, call the CDC Director's Emergency Operations Center (telephone: 770-488-7100), and ask to page the person on call for the Malaria Branch. Recommendations concerning malaria treatment are available at http://www.cdc.gov/malaria/diagnosis_treatment/treatment.htm or by calling the CDC Malaria Hotline. IntroductionMalaria in humans is caused by infection with one or more species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, and P. malariae) that can infect humans. The infection is transmitted by the bite of an infective female Anopheles species mosquito. Malaria remains a devastating global problem, with an estimated 350--500 million cases and 1 million deaths occurring annually, 80% of them in sub-Saharan Africa (1). Forty-nine percent of the world's population lives in areas where malaria is transmitted (e.g., 109 countries in parts of Africa, Asia, the Middle East, Eastern Europe, Central America and South America, the Caribbean, and Oceania) (1). Before the 1950s, malaria was endemic throughout the southeastern United States; an estimated 600,000 cases occurred in 1914 (2). During the late 1940s, a combination of improved housing and socioeconomic conditions, water management, vector-control efforts, and case management interrupted malaria transmission in the United States. Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate the reintroduction of transmission and to monitor patterns of resistance to antimalarial drugs. Anopheline mosquitoes remain seasonally present in all states and territories except Hawaii. The majority of reported cases of malaria diagnosed each year in the United States are imported from regions where malaria transmission is known to occur, although congenital infections and infections resulting from exposure to blood or blood products are also reported in the United States. In addition, occasionally a limited number of cases are reported that might have been acquired through local mosquitoborne transmission (3). State and local health departments and CDC investigate malaria cases acquired in the United States, and CDC analyzes data from imported cases to detect trends in acquisition. This information is used to guide malaria prevention recommendations for international travelers. The signs and symptoms of malaria illness vary, but the majority of patients have fever. Other common symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. The diagnosis of malaria should always be considered for persons with these symptoms who have traveled to an area with known malaria transmission. Malaria also should be considered in the differential diagnosis of persons who have fever of unknown origin, regardless of their travel history. Untreated P. falciparum infections can rapidly progress to coma, renal failure, pulmonary edema, and death. This report summarizes malaria cases reported to CDC among persons with onset of symptoms in 2006. MethodsData SourcesMalaria case data are reported to the National Malaria Surveillance System (NMSS) and the National Notifiable Diseases Surveillance System (NNDSS) (4). Although both systems rely on passive reporting, the numbers of reported cases might differ because of differences in collection and transmission of data. A substantial difference between the data collected in these two systems is that NMSS receives more detailed clinical and epidemiologic data regarding each case (e.g., information concerning the area to which the infected person has traveled). Malaria cases can be reported to CDC through NMSS, NNDSS, or direct consultation with CDC; therefore, cases identified through these various paths are compared and compiled, duplicates are eliminated, and cases are analyzed. This report presents data on the aggregate of cases reported to CDC through all reporting systems. Malaria cases confirmed by blood film or polymerase chain reaction (PCR) among civilians and military personnel are identified by health-care providers or laboratories. Each confirmed malaria case is reported to local or state health departments and to CDC on a uniform case-report form that contains clinical, laboratory, and epidemiologic information.* CDC reviews all report forms received and requests additional information from the provider or the state, if necessary (e.g., when no recent travel to a malarious country is reported). Other cases are reported by telephone to CDC directly by health-care providers, usually when they are seeking assistance with diagnosis or treatment. Information regarding cases reported directly to CDC is shared with the relevant state health department. All cases that have been reported as acquired in the United States are investigated further, including all induced and congenital cases and possible introduced or cryptic cases. Information derived from uniform case-report forms is entered into a database and analyzed annually. A case rate for each country was estimated using estimates of travel volume for U.S. travelers to each country where cases of malaria were acquired and the number of cases among U.S. travelers attributable to each country. Data used to estimate country-specific relative case rates were extrapolated from World Tourism Organization estimates of annual numbers of U.S. travelers to specified countries (5). Estimated relative case rates were determined by dividing the individual country-specific case rate by the median individual country-specific case rate. DefinitionsThe following definitions are used in this report:
This report also uses terminology from recommendations of the World Health Organization (6). Definitions of the following terms are included for reference:
Laboratory DiagnosisEarly and prompt diagnosis of malaria requires that physicians obtain a travel history from every febrile patient. Malaria should be included in the differential diagnosis of every febrile patient who has traveled to a malarious area. If malaria is suspected, a Giemsa-stained film of the patient's peripheral blood should be examined for parasites as soon as possible. Thick and thin blood films must be prepared correctly because diagnostic accuracy depends on blood-film quality and examination of the film by experienced laboratory personnel (7).† Certain reference laboratories and health departments have the capacity to perform PCR diagnosis of malaria, although PCR diagnosis generally is reserved for cases for which blood-film diagnosis of malaria or species determination is inadequate. ResultsGeneral SurveillanceFor 2006, CDC received 1,564 reports of cases of malaria occurring among persons in the United States and its territories, representing a 2.4% increase from the 1,528 cases reported with a date of onset in 2005 (7). A total of 713 cases occurred among U.S. residents, and 217 cases occurred among foreign residents; resident status was not known for 634 cases. The number of cases increased during 1980--2000 among U.S. residents; however, during 2001--2006, the number of cases plateaued (Table 1). Plasmodium SpeciesOf the 1,564 cases reported in 2006, the infecting Plasmodium species was identified and reported in only 991 (63.4%) cases. P. falciparum and P. vivax make up the majority of infections and were identified in 61.8% and 27.7% of infected persons of known species infection, respectively. The number of reported cases of P. falciparum and P. vivax remained relatively stable during 2004--2006 (Table 2). Among 909 cases for which both the region of acquisition and the infecting species were known, P. falciparum accounted for 65.8% of infections acquired in Africa, 22.5% in the Americas, 9.3% in Asia, and 4.5% in Oceania (Table 3). Infections attributed to P. vivax accounted for 4.3% acquired in Africa, 58.3% in the Americas, 61.5% in Asia, and 72.7% in Oceania. Region of Acquisition and DiagnosisAll cases were reported as imported cases. Of 1,140 imported cases for which the region of acquisition was known, 793 (69.6%) were acquired in Africa, 205 (18.0%) in Asia, 120 (10.5%) in the Americas, and 22 (1.9%) in Oceania (Table 3). West Africa accounted for 563 (71.0%) cases acquired in Africa, and India accounted for 121 (59.0%) cases acquired in Asia. In the Americas, a combined total of 82 (68.3%) cases were acquired in Central America and the Caribbean (The Bahamas, Belize, Costa Rica, Dominican Republic, El Salvador, Guatemala, Haiti, Honduras, Jamaica, and Nicaragua), followed by 27 (22.5%) cases in South America (Bolivia, Brazil, Ecuador, Guyana, Peru, and Suriname) and 11 (9.2%) cases in Mexico. Information regarding region of acquisition was missing for 424 (27.7%) imported cases. Among U.S. civilians, a small but steady increase (16%) in cases acquired in Asia occurred from 2005 to 2006. In the United States, six state health departments accounted for 47.8% of the reported cases: California (n = 185), New York City (n = 174), Texas (n = 129), Georgia (n = 90), New Jersey (n = 87), and Illinois (n = 82) (Figure 1). Compared with 2005, the states with the most significant change in number of reported malaria cases in 2006 were Georgia and Alaska. The number of cases reported in Georgia increased from 54 cases in 2005 to 90 cases in 2006, and Alaska reported an increase from eight cases in 2005 to 23 cases in 2006; all cases occurred among U.S. military personnel, all of whom had traveled to Afghanistan. Imported Malaria by Resident StatusOf 930 imported malaria cases of known resident status, 713 (76.7%) occurred among U.S. residents, and 217 (23.3%) occurred among residents of other countries. Of the 713 imported cases, 511 (71.7%) were acquired in Africa, 116 (16.3%) were acquired in Asia, and 42 (5.9%) were acquired in Central America and the Caribbean (Table 4). Of the 217 imported cases among foreign residents, 131 (60.4%) were acquired in Africa. Of patients with foreign cases for whom purpose of travel was known, 76 (58%) identified as being a recent immigrant or refugee, and 22 (17%) reported visiting friends and relatives in the United States. Relative Case Rates Among U.S. ResidentsIn 2006, the countries with the lowest estimated case rates of malaria among U.S. travelers (among countries that reported cases) were The Bahamas and Jamaica, both of which had been considered nonendemic countries but experienced malaria outbreaks in 2006 (Figure 2). Examples of other countries with low estimated relative case rates include Mexico, Vietnam, Costa Rica, and Thailand. For many of these countries, malaria risk areas are concentrated in small parts of the country. Examples of countries with estimated relative case rates in the middle range include India, Honduras, Haiti, and Kenya, which have malaria transmission occurring more homogenously throughout the country. Estimated relative case rates were highest in countries in West Africa and Oceania, including Nigeria, Ghana, Papua New Guinea, and Vanuatu. These high estimated case rates not only reflect widespread transmission areas but also likely reflect higher transmission intensity. Interval Between Arrival and IllnessBoth the interval between date of arrival in the United States and onset of illness and the infecting Plasmodium species were known for only 622 (39.8%) of the imported malaria cases (Table 5). Symptoms began before arrival in the United States for 40 (6.4%) persons and after arrival for 582 (93.6%) persons. Clinical malaria occurred <30 days after arrival in 384 (89.5%) of the 429 persons with P. falciparum cases and in 69 (54.3%) of the 127 P. vivax cases (Table 5). Six (1.0%) of 622 persons became ill with an infection with P. vivax or P ovale >1 year after returning to the United States. Imported Malaria Among U.S. Military PersonnelIn 2006, 50 cases of imported malaria were reported among U.S. military personnel. Information on infecting species was known for 38 cases: 33 cases of P. vivax, four cases of P. falciparum, and one case of P. malariae. Among the 38 cases with known infecting species, 33 patients had reported taking chemoprophylaxis, of whom 19 (57.5%) had taken the correct CDC-recommended antimalarial drug for the specific region of travel; only one patient reported adherence to the prescribed drug regimen. These cases were reported by state health departments and do not include all cases reported through malaria surveillance activities conducted by the U.S. Department of Defense. Chemoprophylaxis Use Among U.S. CiviliansInformation concerning chemoprophylaxis use and travel area was known for 602 (90.8%) of the 663 U.S. civilians who had imported malaria. Of these 602 patients, 405 (67.3%) had not taken any chemoprophylaxis. Of the 197 patients who did report taking malaria chemoprophylaxis, 58 (29.4%) had not taken a CDC-recommended drug for the area visited, whereas 131 (20.9%) had taken a CDC-recommended drug (7). Data for the specific drug taken were missing for the remaining eight (4.1%) travelers. A total of 58 (44.3%) patients receiving CDC-recommended chemoprophylaxis reported taking mefloquine; 36 (27.5%) had taken doxycycline; 25 (19.1%) had taken atovaquone-proguanil; and eight (6.1%) who had traveled only in areas where chloroquine-resistant malaria has not been documented had taken chloroquine. Of the 131 persons taking a CDC-recommended malaria chemoprophylaxis for the travel region, only 52 (40.0%) reported adherence to the prescribed drug regimen. Malaria Infection After Recommended Prophylaxis UseA total of 220 patients (including 136 U.S. civilians, 41 persons in the U.S. military, eight foreign residents, and 35 persons for whom information regarding status was missing) contracted malaria after taking a recommended antimalarial drug for chemoprophylaxis. Of these, 76 (34.5%) reported complete adherence with the drug regimen, and 104 (47.3%) reported nonadherence; adherence was unknown for the remaining 40 (18.2%). Information regarding infecting species was available for 178 (80.9%) patients who had taken a recommended antimalarial drug; the infecting species was undetermined for the remaining 42 patients. Cases Caused by P. vivax or P. ovale Among the 220 patients who received a diagnosis of malaria after recommended chemoprophylaxis use, 70 (31.8%) had cases that were caused by P. vivax, and 10 (4.5%) had cases caused by P. ovale. Among the 80 total cases of P. vivax or P. ovale, 41 (51.2%) occurred >45 days after arrival in the United States. These cases were consistent with relapsing infections and do not indicate primary prophylaxis failures. Information on 19 cases was insufficient (i.e., missing data regarding symptom onset or return date) to assess whether the infection was a relapse infection. A total of 20 cases occurred <45 days after the patient returned to the United States; 10 each caused by P. vivax and P. ovale. Nine of the 20 patients did not adhere to their malaria chemoprophylaxis regimen; information regarding drug regimen adherence was missing for four cases. Seven patients reported adherence with an antimalarial chemoprophylaxis regimen. Of these seven, three patients had traveled to Africa, two of whom reported taking atovaquone-proguanil as malaria chemoprophylaxis and one who reported taking doxycycline. Two patients had traveled to Asia; one had traveled to India and had taken primaquine, and one had traveled to Iraq and taken mefloquine for chemoprophylaxis. The remaining two patients had traveled to Central America and South America; one patient had traveled to Peru and took doxycycline, and one had traveled to Honduras and taken mefloquine for chemoprophylaxis. Possible explanations for these cases include inappropriate dosing, unreported nonadherence to the regimen, malabsorption of the drug, or emerging parasite resistance. Cases Caused by P. falciparum and P. malariae Ninety-four cases of malaria were reported among persons who had taken a recommended antimalarial drug for chemoprophylaxis, including 78 cases of P. falciparum and 16 of P. malariae. Of the 78 P. falciparum cases among those who reported taking a recommended antimalarial drug, all except one case was acquired in Africa. Forty-three (55.1%) patients reported nonadherence to the antimalarial drug regimen; adherence information was not available for 14 cases. In 21 (26.9%) cases, patients reported adherence with malaria chemoprophylaxis, all of whom had traveled to Africa. Fourteen patients reported taking mefloquine, three doxycycline, one primaquine, and three took atovaquone-proguanil. Of the 16 P. malariae cases, 11 were acquired in Africa, four in Asia, and one in the Caribbean. Five patients reported nonadherence to the antimalarial drug regimen, and no adherence information was available for five cases. For the remaining six cases, patients reported adhering to the drug regimen; three took mefloquine, two took doxycycline, and one took atovaquone-proguanil. Cases of Mixed-Species Infection Among the 220 patients who had taken a recommended malaria chemoprophylaxis, four had a mixed Plasmodium species infection. Three patients had traveled to Africa, two of whom had a mixed infection of P. vivax and P. falciparum, and one who had P. vivax and P. ovale infections. All three patients had taken atovaquone-proguanil for malaria chemoprophylaxis; however, one had not completed the drug regimen, and no adherence information was available for the remaining two. The patient with the fourth mixed-infection case (P. ovale and P. falciparum) had traveled to Papua New Guinea and also did not complete the malaria chemoprophylaxis drug regimen. Purpose of TravelPurpose of travel to areas in which malaria is endemic was reported for 617 (86.5%) of the 713 U.S. civilians with imported malaria. Though travelers could report multiple reasons for travel, the largest proportion (50.9%) represented persons who had visited friends or relatives in malarious areas; the second and third highest proportions, 9.9% and 7.4%, represented persons who had traveled as missionaries or as tourists, respectively (Table 6). Malaria in ChildrenOf the 1,445 cases for whom age was known, 280 (19.4%) cases occurred in persons aged <18 years. Among these, 113 (40.4%) cases occurred among U.S. civilian children, 78 (27.9%) occurred among children of foreign citizenship, and for 89 (31.8%) cases, resident status was unknown. Ninety-four (83.1%) of the cases among U.S. civilian children for whom country of exposure was known were attributable to Africa. Of the 113 cases among U.S. civilian children, six (5.3%) of the children were aged <24 months, 24 (21.2%) were aged 24--59 months, 40 (35.4%) were aged 5--12 years, and 24 (21.2%) were aged 13--17 years. Among 75 patients for which reason for travel was known, 74 were reported as visiting friends and relatives; the remaining patient was reported as a being a tourist. Among the 88 cases for whom chemoprophylaxis information was known, 30 (34%) patients reported taking chemoprophylaxis, of whom 18 (60%) had taken a correct regimen; only three (16.7%) reported complete adherence. Malaria During PregnancyA total of 17 cases of malaria were reported among pregnant women in 2006, representing 3.4% of cases among women. Eight (47.0%) of the 17 cases occurred among U.S. civilians, five of whom had traveled to Africa and three of whom had traveled to countries in Central America and South America. The five women who traveled to Africa were reported as visiting friends and relatives; the three women who traveled to Central America and South America were reported as being tourists. Of the eight cases of malaria reported among U.S. civilian pregnant women, only one (12.5%) woman reported taking malaria chemoprophylaxis; however, she reported taking an inappropriate medication. No information was available on the birth outcomes of the pregnant women. Deaths Attributed to MalariaSix deaths attributable to malaria were reported in 2006 and are described in the following case reports: Case 1 On March 20, a man aged 47 years from Thailand arrived in New York City and developed fever and lethargy. He had a medical history of alcoholic cirrhosis, ascites, spontaneous bacterial peritonitis, and diabetes. On March 21, the patient was admitted to the hospital and had symptoms of a chronically ill person, with fever, jaundice, and a distended abdomen. The initial diagnosis was hepatic encephalopathy and spontaneous bacterial peritonitis; peripheral blood smears were ordered to determine whether a concurrent malaria infection was present. The patient was intubated, received mechanical ventilation, and was treated with ceftriaxone, ampicillin/sulbactam, clindamycin, and dexamethasone. The blood smear was positive for P. malariae by microscopy, which was subsequently confirmed by PCR. Doxycycline and quinidine gluconate were added to the regimen within 24 hours of admission. The patient experienced renal failure and died on March 25, 2006. Case 2 On March 30, a woman aged 65 years returned to Arizona from a 17-day tour of Kenya and Tanzania. Malaria prophylaxis had been prescribed, but the entire regimen was not completed. On June 7, the woman experienced fever and chills. On June 9, she sought treatment at an emergency room; she was evaluated, received intravenous fluids, and was sent home. On June 12, the patient returned to the hospital with the same symptoms as well as nausea and syncope. A peripheral blood film revealed P. falciparum infection; the patient was administered oral quinine and doxycycline. On June 13, she experienced a decreased level of consciousness, necessitating intubation and mechanical ventilation. She died the same evening. Case 3 On June 29, a girl aged 2 years who recently had emigrated from Nigeria was hospitalized in Boston, Massachusetts, for fever, vomiting, diarrhea, and scleral icterus. Peripheral blood films obtained on admission were positive for P. falciparum, with 1.4% parasitemia; she was administered oral atovaquone-proguanil. On June 30, her parasitemia increased to 26.7%, and she was transferred to the pediatric intensive care unit for an exchange transfusion and continuous quinidine infusion plus intravenous clindamycin. By July 2, the parasitemia had resolved, but the patient experienced acute respiratory distress syndrome. The patient was intubated and mechanical ventilation was initiated; she died on July 3, 2006. Case 4 On July 28, a man aged 59 years returned from a month-long trip to Ghana. The patient also traveled regularly to India twice per year for mission work; his most recent trip had been in January 2006. He did not take malaria prophylaxis routinely while in India and did not take malaria prophylaxis while traveling to Africa. The evening he returned to the United States, he experienced a high fever and went to an urgent care center, where he received a diagnosis of community-acquired pneumonia and was treated with azithromycin. The patient's symptoms persisted and progressed to include vomiting and melena. He sought medical attention again on August 1; a peripheral blood film revealed P. falciparum with 10% parasitemia. He was administered oral mefloquine and transferred to a hospital intensive care unit (ICU), where he was treated with quinine and doxycycline. Within 24 hours, he experienced respiratory distress and was intubated. Quinidine gluconate was recommended, and exchange transfusion was initiated; however the patient died before quinidine gluconate could be administered. The patient died on August 3, 2006. Case 5 On October 17, a woman aged 75 years who was a resident of India arrived in Arizona to attend a family wedding. On October 19, she was taken to the hospital because of fever, disorientation, and decreased level of consciousness. Her medical history included a neurogenic bladder, necessitating self-catheterization, and cerebral malaria. The initial diagnosis was pyelonephritis, and she was treated with intravenous ceftriaxone in the ICU. On October 20, a peripheral blood film revealed P. falciparum; quinine and doxycycline were added to the treatment regimen. The patient experienced renal failure and acute respiratory distress syndrome and was intubated on the October 23. She experienced a nosocomial blood stream infection, and antibiotics were continued; however, her condition continued to deteriorate. The patient was discharged to hospice care and died on November 11, 2006. Case 6 Case 6 was reported as case 2 in the CDC malaria surveillance report published in 2007 (7). However, the actual date of onset was April 19, 2006, not 2005 as previously reported. On April 19, a man aged 55 years was taken to an ED with a 4-day history of fever, emesis, and epigastric pain. He was a resident of the United States but had traveled to Uganda, his country of origin, for 3 months and had returned on April 12. He had not taken malaria prophylaxis. On admission, he had sinus tachycardia and a temperature of 100.3ºF (37.9ºC). Routine laboratory analysis was significant only for thrombocytopenia (platelet count: 19,000/µL). A differential diagnoses list was generated, including malaria, dengue fever, and Chikungunya fever; no additional evaluations were performed. The patient's symptoms improved with antiemetics, normal saline, and pain control. He was discharged with a tentative diagnosis of dengue fever. Four days later, on April 23, he died abruptly. Samples sent to CDC were positive for P. falciparum by PCR but negative for other suspected pathogens. DiscussionA total of 1,564 cases of malaria were reported to CDC for 2006, representing an increase from the total number of cases reported in 2005. The number of cases with no information regarding residential status or clinical information increased from 2005 to 2006, from 325 unidentified cases to 634 unidentified cases (7). Excluding the cases with no information on residence status, the percentage of U.S. resident cases was relatively stable from 2000 to 2006. Although during 2002--2006, the number of cases acquired from specific regions overall was stable, the proportion of cases from Asia increased slightly but steadily. Flux in the number of cases acquired in a specific region can be affected by many factors, including the amount of transmission occurring in the region, adherence to preventive measures (e.g., mosquito avoidance and chemoprophylaxis) by travelers, the purpose of travel that predominates in that country (e.g., business travel or adventure travel), and the volume of travel to those countries. Of the 1,564 imported cases, 634 (40.5%) cases did not have information available regarding residential status, and 424 (27.1%) did not have information regarding travel history. An increase in the number of cases that do not have residential and clinical information available decreases the accuracy with which the data reflect trends in malaria surveillance in the United States. Vigilance needs to be exercised by local and state health departments, health-care providers, and other health personnel to provide accompanying information regarding malaria cases when submitting cases through the various reporting systems to CDC. In the Caribbean region, the endemic transmission of malaria ended in the mid-1960s, except in the island of Hispaniola, which includes the countries of Dominican Republic and Haiti (8). In 2006, three cases of malaria in the United States were reported from the Caribbean region; two in U.S. travelers and one in a foreign visitor. Two of the three cases were acquired from Jamaica and one from The Bahamas (Great Exuma Island); all were caused by P. falciparum. Although these countries were not considered risk areas for malaria, the islands remained at risk for reintroduction because of the tropical climate, presence of viable vectors, and close proximity to countries where malaria is still endemic. As a result, CDC issued recommendations on June 16, 2006, and December 4, 2006, for chloroquine chemoprophylaxis for persons traveling to Great Exuma, The Bahamas, and Kingston, Jamaica, respectively. Through several interventions, including active case finding and treatment and mosquito-control strategies, the outbreak in Great Exuma seemed to be controlled. CDC rescinded chemoprophylaxis recommendations for travelers to Great Exuma on September 19, 2006 (9). However, additional cases have occurred in travelers, and chemoprophylaxis recommendations have been reinstated. In Jamaica, similar strategies to contain the outbreak have been used; however, rare cases continue to be identified in Kingston.§ Fortunately, these occasional cases do not present a substantial ongoing risk to travelers, and routine chemoprophylaxis is no longer recommended. Both of these outbreaks demonstrate the importance of the constant vigilance of health-care providers and rapid response by public health officials for effective containment strategies to avoid widespread reintroduction of malaria in nonendemic areas. U.S. military personnel usually are on long-term prophylaxis for malaria to adequately protect them for the duration of their deployment in malaria-endemic regions. In regions where P. vivax or P. ovale infections predominate, primaquine is recommended in addition to the chemoprophylactic regimen to prevent relapsing malaria infection (10). The U.S. Army recommends that U.S. soldiers being deployed in regions where P. vivax infections predominate should take mefloquine or doxycycline as the primary prophylactic drug, followed by primaquine for 2 weeks before their return to the United States (11,12). In 2006, 19 of 25 soldiers who had long-term (>1 year) deployments in Afghanistan had not taken primaquine terminal prophylaxis after their course of doxycycline as prescribed by the U.S. Army. Of the remaining six soldiers who had taken both doxycycline and primaquine chemoprophylaxis, only one soldier reported adherence to the entire regimen. One purpose of malaria surveillance is to identify prophylaxis failures that might indicate emergence of drug resistance. However, approximately 82% of imported malaria cases among U.S. residents for whom information regarding prophylaxis use was available occurred among persons who were either not taking prophylaxis or were taking a non-CDC recommended prophylaxis regimen. Based on available information, the majority of patients who apparently received an appropriate medication and had onset of symptoms within 45 days, reported nonadherence with their chemoprophylactic regimen or provided insufficient information to make a determination regarding adherence. Because CDC does not actively seek serum drug levels from patients who report adherence with a recommended regimen, differentiating among inaccurate reporting of adherence, malabsorption of the antimalarial drug, and emerging drug resistance is not possible. No conclusive evidence indicates a single national or regional source of infection in this group of patients or the failure of a particular chemoprophylactic regimen. Health-care providers are encouraged to contact CDC quickly when chemoprophylaxis failure is suspected to enable CDC to measure serum drug levels of the antimalarial drugs in question and parasite from the patient to evaluate for possible drug resistance. Of the six fatal cases that occurred in the United States in 2006, four of the patients reported taking no chemoprophylaxis while traveling to areas where malaria is endemic; information on chemoprophylaxis use for the remaining two cases was not available. One patient substantially delayed seeking care, five had substantial delays in establishing a diagnosis, and three patients experienced a delay in receiving appropriate treatment. These findings underscore the importance of patients adhering to correct chemoprophylaxis and promptly seeking medical care if symptoms develop, as well as the importance of physicians considering malaria in the differential diagnosis of fever in a person who has returned from travel. A previous review of deaths attributed to malaria in the United States indicated that failure to take or adhere to recommended antimalarial chemoprophylaxis, promptly seek medical care for posttravel illness, and promptly diagnose and treat suspected malaria all contributed to fatal outcomes (13). As in previous years, people who traveled to visit friends and relatives experienced the majority of malaria cases in 2006. Foreign-born U.S. civilians need to be aware that acquired immunity wanes quickly when malaria exposure is interrupted and that they need to take prophylaxis when returning to malarious areas. Additionally, children of foreign-born U.S. civilians who are born in the United States are not immune to malaria and are highly vulnerable to infection (14). In this summary, approximately three fourths of the children with malaria whose reason for travel was to visit friends and relatives abroad had not been taking any chemoprophylaxis or had been taking an incorrect medication for chemoprophylaxis. Seventeen cases were reported in pregnant women, a 21% increase from 2005. Among U.S. civilians who were pregnant, only one of eight women (12.5%) reported taking chemoprophylaxis. This proportion is much lower than the percentage of total U.S. civilians with malaria who took chemoprophylaxis. Malaria during pregnancy among women who are not immune poses a high risk for both maternal and perinatal morbidity and mortality (15). Pregnant travelers should be counseled to avoid travel to malarious areas. If deferral of travel is impossible, these women should be informed that the risks from malaria greatly outweigh those associated with prophylaxis and that safe chemoprophylaxis regimens are available and should be emphasized. Information for pregnant travelers is available at http://wwwn.cdc.gov/travel/contentmalariapregnantpublic.aspx. Signs and symptoms of malaria are often nonspecific, but fever usually is present. Other symptoms include headache, chills, increased sweating, back pain, myalgia, diarrhea, nausea, vomiting, and cough. For prompt diagnosis, malaria must be included in the differential diagnosis of illness in a febrile patient with a history of travel to a malarious area. Clinicians should ask all febrile patients for a travel history, including international visitors, immigrants, refugees, migrant laborers, and other international travelers. Prompt treatment of suspected malaria is essential because persons with P. falciparum infection are at risk for experiencing life-threatening complications soon after onset of illness. Ideally, therapy for malaria should be initiated immediately after the diagnosis has been made. Treatment should be determined on the basis of the infecting Plasmodium species, the probable geographic origin of the parasite, the parasite density, and the patient's clinical status (15). If malaria is suspected but cannot be confirmed or malaria is confirmed but species determination is not possible, antimalarial treatment that is effective against P. falciparum should be initiated. Resistance of P. falciparum to chloroquine occurs worldwide, with the exception of a limited number of geographic regions (e.g., Central America). Therefore, therapy for presumed P. falciparum malaria should entail the use of a drug effective against such resistant strains (16). The findings in this report are subject to at least two limitations. First, although malaria is a notifiable disease in the United States, malaria case counts are obtained through passive surveillance systems and from individual reporting from health-care professionals treating persons with malaria. Therefore, only cases that are diagnosed by health-care or laboratory workers and reported to state and local health departments and to CDC are included, possibly resulting in an underestimate of disease incidence. Second, the completeness of reporting to the surveillance system might vary by reporting group and by year, which limits the amount of information that can be learned from each case. Health-care providers should be familiar with prevention, recognition, and treatment of malaria and are encouraged to consult appropriate sources for malaria prevention and treatment recommendations (Table 7). Physicians seeking assistance with the diagnosis or treatment of patients with suspected or confirmed malaria should call CDC's Malaria Branch (telephone: 770-488-7788; Monday--Friday, 8:00 AM--4:30 PM EST); call CDC's Emergency Operations Center (telephone: 770-488-7100) during evenings, weekends, and holidays (ask to page person on call for Malaria Branch); or visit CDC's website at http://www.cdc.gov/malaria/diagnosis_treatment/treatment.htm. These resources are intended for use by health-care providers only. Detailed recommendations for the public for preventing malaria are available online at http://wwwn.cdc.gov/travel/contentdiseases.aspx#malaria. In addition, biannually, CDC publishes recommendations in Health Information for International Travel (commonly referred to as The Yellow Book) (17), which is available and updated frequently on CDC's website at http://wwwn.cdc.gov/travel; The Yellow Book also can be purchased online from Elsevier at http://www.elsevierhealth.com or by telephone (800-545-2522). CDC provides assistance for diagnostic parasitology through DPDx (Laboratory Identification of Parasites of Public Health Concern), a project developed and maintained by CDC's Division of Parasitic Diseases. DPDx (available at http://www.dpd.cdc.gov/dpdx) provides free Internet-based laboratory diagnostic assistance (i.e., telediagnosis) to laboratorians and pathologists in suspected parasitic disease cases, such as malaria. Digital images captured from diagnostic specimens can be submitted for consultation through e-mail. Telediagnosis assistance by CDC is available during regular business hours. Because laboratories can transmit images to CDC and obtain a rapid response (with average time ranging from minutes to several hours) to their inquiries, this system allows efficient diagnosis of challenging cases and rapid dissemination of information. As of January 2008, approximately 49 public health laboratories in 46 states and Puerto Rico had the ability to perform telediagnosis. Implementation of telediagnosis at public health laboratories receives full assistance from CDC, including training of personnel in digital imaging techniques. The DPDx website also contains reference material with images, text, and videos on approximately 100 different species of parasites with information (including laboratory diagnosis, geographic distribution, clinical features, treatment, and life cycles) available for each parasite. AcknowledgmentsThis report is based, in part, on data reported from state, territorial, and local health departments; health-care providers; and laboratories. References
* Malaria case-report surveillance form available at http://www.cdc.gov/malaria/clinicians.htm#case. † To obtain confirmatory diagnosis of blood films from questionable cases and to obtain appropriate treatment recommendations, contact either the state or local health department or CDC's Malaria Branch (770-488-7788). § Additional information on the malaria outbreak in Jamaica is available from the World Health Organization at http://www.who.int/csr/don/2007_02_09/en/index.html. Table 1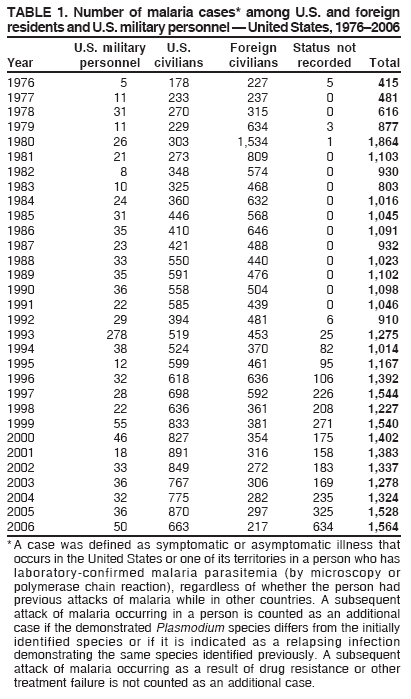 Return to top. Figure 1 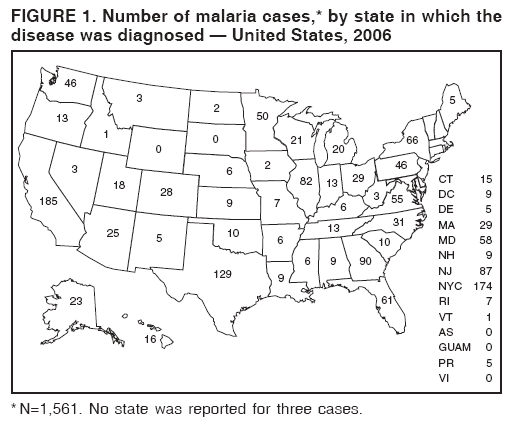 Return to top. Table 2 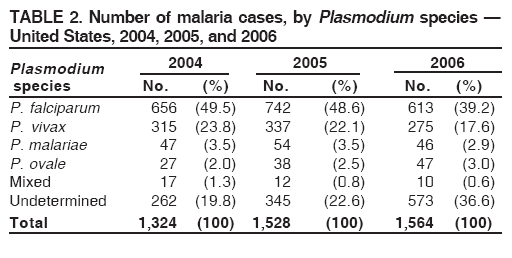 Return to top. Figure 2 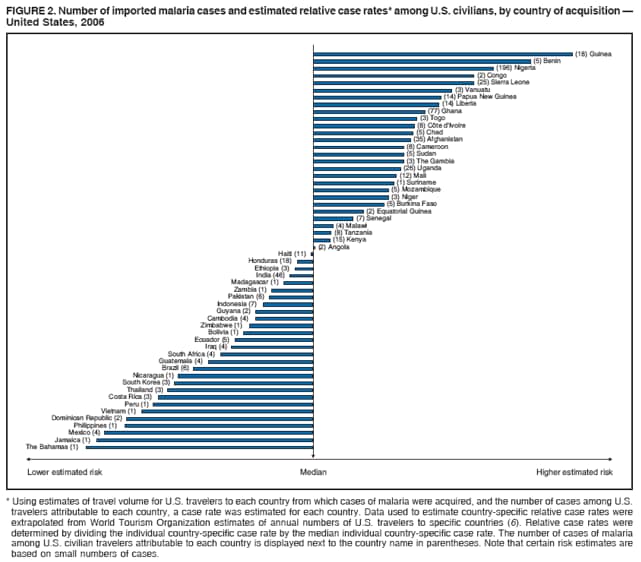 Return to top. Table 3 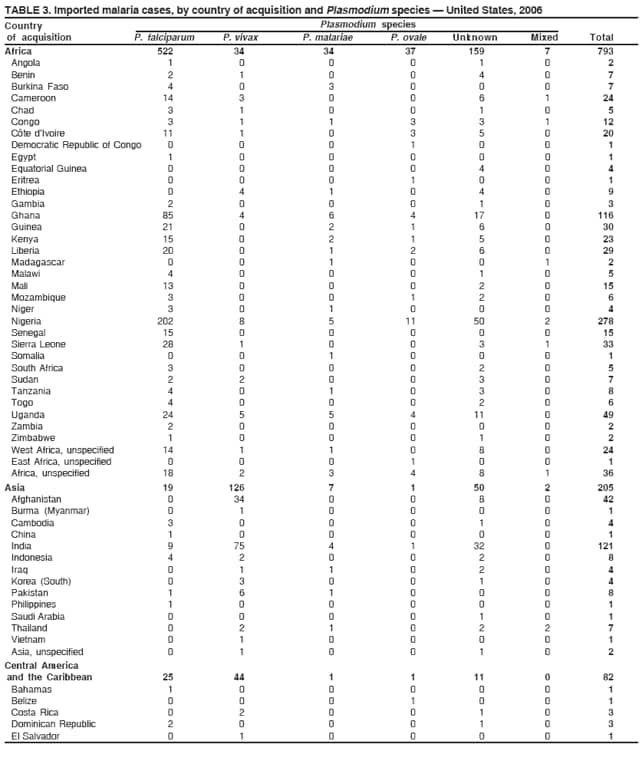 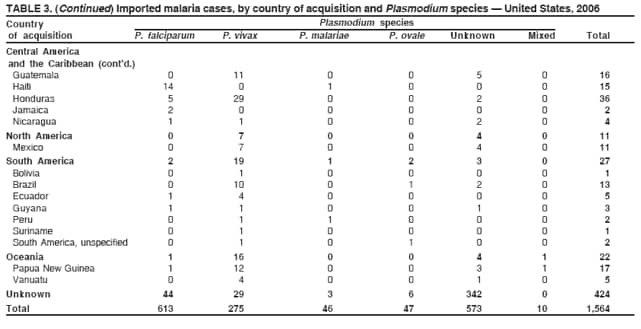 Return to top. Table 4 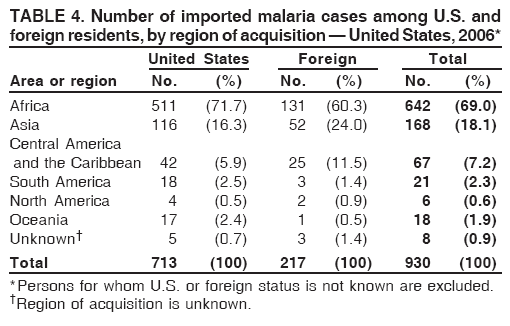 Return to top. Table 5 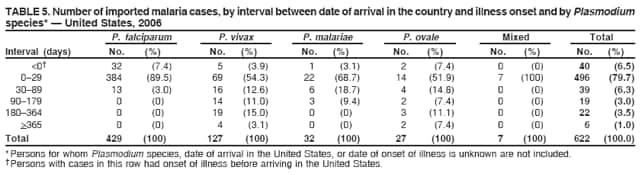 Return to top. Table 6 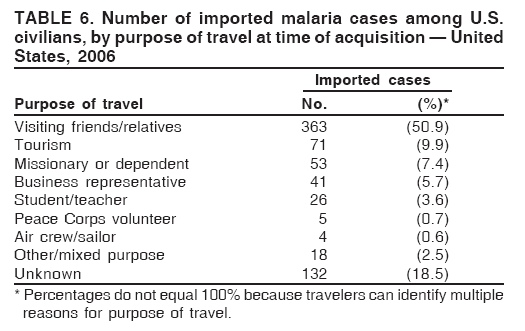 Return to top. Table 7 ![TABLE 7. Sources for malaria prophylaxis, diagnosis, and treatment recommendations
Type of
information Source Availability Telephone number, website, or e-mail address
Prophylaxis CDC Travelers’ Health website (includes 24 hours/day http://www.cdc.gov/travel
online access to Health Information for
International Travel [The Yellow Book])
Prophylaxis Health Information for International Travel Order from: 800-545-2522 or
(The Yellow Book) Elsevier, Health Sciences http://www.elsevier.com
Division Order Fulfillment
11830 Westline Industrial Drive
St. Louis, MO 63146
Prophylaxis CDC Malaria Risk Map 24 hours/day http://www.cdc.gov/malaria/features/risk_map.htm
Diagnosis CDC, Division of Parasitic Diseases website: 24 hours/day http://www.dpd.cdc.gov/dpdx
Laboratory Identification of Parasites of
Public Health Concern (DPDx)
Diagnosis CDC, Division of Parasitic Diseases diagnostic Order by e-mail from CDC, dpdx@cdc.gov
CD-ROM (DPDx) Division of Parasitic
Diseases
Treatment* CDC Malaria Branch 8:00 A.M.–4:30 P.M. EST, 770-488-7788*
Monday–Friday
Treatment* CDC Malaria Branch 4:30 P.M.–8:00 A.M. EST, 770-488-7100* (Telephone number for CDC
on weekdays; all day on Director’s Emergency Operations Center. Ask a
weekends and holidays staff member to page the person on call for the
Malaria Branch.)
http://www.cdc.gov/malaria/diagnosis_treatment/
treatment.htm.
* Telephone number is for health-care professionals only.](figures/s705a2t7.gif) Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/4/2008 |
|||||||||
|