 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Malaria Surveillance --- United States, 2002Snehal Shah, M.D.1,2 AbstractProblem/Condition: Malaria is caused by any of four species of intraerythrocytic protozoa of the genus Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, or P. malariae). These parasites are transmitted by the bite of an infective female Anopheles species mosquito. The majority of malaria infections in the United States occur among persons who have traveled to areas with ongoing transmission. In the United States, cases can occur through exposure to infected blood products, by congenital transmission, or by local mosquitoborne transmission. Malaria surveillance is conducted to identify episodes of local transmission and to guide prevention recommendations for travelers. Period Covered: This report covers cases with onset of illness in 2002. Description of System: Malaria cases confirmed by blood film are reported to local and state health departments by health-care providers or laboratory staff. Case investigations are conducted by local and state health departments, and reports are transmitted to CDC through the National Malaria Surveillance System (NMSS). Data from NMSS serve as the basis for this report. Results: CDC received reports of 1,337 cases of malaria with an onset of symptoms in 2002 among persons in the United States or one of its territories. This number represents a decrease of 3.3% from the 1,383 cases reported for 2001. P. falciparum, P. vivax, P. malariae, and P. ovale were identified in 52.3%, 25.4%, 2.8%, and 2.8% of cases, respectively. Eleven patients (0.8% of total) were infected by >2 species. The infecting species was unreported or undetermined in 213 (15.9%) cases. Compared with 2001, the number of reported malaria cases acquired in Asia (n = 171) and Africa (n = 903) increased by 4.3% and 1.9%, respectively, whereas the number of cases acquired in the Americas (n = 141) decreased by 41.2%. Of 849 U.S. civilians who acquired malaria abroad, 317 (37.3%) reported that they had followed a chemoprophylactic drug regimen recommended by CDC for the area to which they had traveled. Five patients became infected in the United States, one through congenital transmission, one probable transfusion-related, and three whose infection cannot be linked epidemiologically to secondary cases. Eight deaths were attributed to malaria. All deaths were caused by P. falciparum. Interpretation: The 3.3% decrease in malaria cases in 2002, compared with 2001, resulted primarily from a marked decrease in cases acquired in the Americas, but this decrease was offset somewhat by an increase in the number of cases acquired in Africa and Asia. This limited decrease probably represents year-to-year variation in malaria cases, but also could have resulted from local changes in disease transmission, decreased travel to malaria-endemic regions, fluctuation in reporting to state and local health departments, or an increased use of effective antimalarial chemoprophylaxis. In the majority of reported cases, U.S. civilians who acquired infection abroad were not on an appropriate chemoprophylaxis regimen for the country in which they acquired malaria. Public Health Actions: Additional information was obtained concerning the eight fatal cases and the five infections acquired in the United States. Persons traveling to a malarious area should take one of the recommended chemoprophylaxis regimens appropriate for the region of travel, and travelers should use personal protection measures to prevent mosquito bites. Any person who has been to a malarious area and who subsequently experiences a fever or influenza-like symptoms should seek medical care immediately and report their travel history to the clinician; investigation should include a blood-film test for malaria. Malaria infections can be fatal if not diagnosed and treated promptly. Recommendations concerning malaria prevention can be obtained from CDC by calling the Malaria Hotline at 770-488-7788 or by accessing CDC's Internet site at http://www.cdc.gov/travel. IntroductionMalaria in humans is caused by infection with one or more of four species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, and P. malariae). The infection is transmitted by the bite of an infective female Anopheles species mosquito. Malaria infection remains a devastating global problem, with an estimated 300--500 million cases occurring annually (1). Forty-one percent of the world's population lives in areas where malaria is transmitted (e.g., parts of Africa, Asia, the Middle East, Central and South America, Hispaniola, and Oceania) (1), and 700,000--2.7 million persons die of malaria each year, 75% of them African children (2). Before the 1950s, malaria was endemic throughout the southeastern United States; an estimated 600,000 cases occurred in 1914 (3). During the late 1940s, a combination of improved housing and socioeconomic conditions, water management, vector-control efforts, and case management was successful at interrupting malaria transmission in the United States. Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate the reintroduction of transmission and to monitor patterns of antimalarial drug resistance. Anopheline mosquitos remain seasonally present in all states except Hawaii. The majority of cases of malaria each year diagnosed in the United States have been imported from regions of the world where malaria transmission is known to occur, although congenital infections and infections resulting from exposure to blood or blood products are also reported in the United States. In addition, a limited number of cases are reported that might have been acquired through local mosquitoborne transmission (4). State and local health departments and CDC investigate malaria cases acquired in the United States, and CDC analyzes data from imported cases to detect trends in acquisition. This information is used to guide malaria prevention recommendations for international travelers. For example, an increase in P. falciparum malaria among U.S. travelers to Africa, an area with increasing chloroquine resistance, prompted CDC to change the recommended chemoprophylaxis regimen from chloroquine to mefloquine in 1990 (5). The signs and symptoms of malaria illness are varied, but the majority of patients experience fever. Other common symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. The diagnosis of malaria should be considered for persons who experience these symptoms and who have traveled to an area with known malaria transmission. Malaria should also be considered in the differential diagnoses of persons who experience fevers of unknown origin, regardless of their travel history. Untreated P. falciparum infections can rapidly progress to coma, renal failure, pulmonary edema, and death. Asymptomatic parasitemia can occur, most commonly among persons who have been long-term residents of malarious areas. This report summarizes malaria cases reported to CDC with onset of symptoms in 2002. MethodsData SourcesMalaria case data are reported to the National Malaria Surveillance System (NMSS) and the National Notifiable Diseases Surveillance System (NNDSS) (6). Both systems rely on passive reporting, and the numbers of reported cases might differ because of differences in collection and transmission of data. A substantial difference in the data collected in these two systems is that NMSS receives more detailed clinical and epidemiologic data regarding each case (e.g., information concerning the area to which the infected person has traveled). This report presents only data regarding cases reported to NMSS. Cases of blood-film--confirmed malaria among civilians and military personnel are identified by health-care providers or laboratories. Each slide-confirmed malaria case is reported to local or state health departments and to CDC on a uniform case report form that contains clinical, laboratory, and epidemiologic information. CDC staff review all report forms when received and request additional information from the provider or the state, if necessary (e.g., when no recent travel to a malarious country is reported). Reports of other cases are telephoned to CDC directly by health-care providers, usually when they are seeking assistance with diagnosis or treatment. Cases reported directly to CDC are shared with the relevant state health department. All cases that have been acquired in the United States are investigated, including all induced and congenital cases and possible introduced or cryptic cases. Information derived from uniform case report forms is entered into a database and analyzed annually. DefinitionsThe following definitions are used in this report:
This report also uses terminology derived from the recommendations of the World Health Organization (7). Definitions of the following terms are included for reference:
--- Indigenous. Mosquitoborne transmission of malaria in a geographic area where malaria occurs regularly.
Microscopic Diagnosis of MalariaThe early and prompt diagnosis of malaria requires that physicians obtain a travel history from every febrile patient. Malaria should be included in the differential diagnosis of every febrile patient who has traveled to a malarious area. If malaria is suspected, a Giemsa-stained film of the patient's peripheral blood should be examined for parasites. Thick and thin blood films must be prepared correctly because diagnostic accuracy depends on blood-film quality and examination by experienced laboratory personnel* (Appendix). ResultsGeneral SurveillanceFor 2002, CDC received 1,337 malaria case reports occurring among persons in the United States and its territories, representing a 3.3% decrease from the 1,383 cases reported with a date of onset in 2001 (8). This incidence is the sixth highest number of reported cases since 1980 and represents the second highest number of U.S. civilian cases reported in the previous 30 years (Table 1). In 2002, a total of 849 cases occurred among U.S. civilians, compared with 891 cases reported for 2001; the number of cases among foreign civilians decreased from 316 cases to 272 (Table 1). During 1997--2001, an increase in cases among U.S. civilians has occurred, but cases among foreign civilians have decreased (Figure 1). Cases among U.S. military personnel increased from 18 to 33 in 2002. For 183 cases, information was insufficient to determine civilian or military status. Plasmodium SpeciesThe infecting species of Plasmodium was identified in 1,124 (84.1%) of the cases reported in 2002. P. falciparum and P. vivax were identified in blood films from 52.3% and 25.4% of infected persons, respectively (Table 2). The 699 P. falciparum cases reported for 2002 represented a 0.8% increase from the 693 cases in 2001, and the number of P. vivax infections decreased by 11.9% (from 385 in 2001 to 339 in 2002). Among 1,044 cases in which both the region of acquisition and the infecting species were known, 81.7 % of infections acquired in Africa were attributed to P. falciparum; 9.5% were attributed to P. vivax. The converse was true of infections acquired in the Americas and Asia: 70.0% and 82.8% were attributed to P. vivax, and only 26.2% and 11.5% were attributed to P. falciparum, respectively. Region of Acquisition and DiagnosisAll but five reported cases (n = 1,332) were imported. Of 1,252 imported cases in which the region of acquisition was known, the majority (72.1%; n = 903) were acquired in Africa; 13.7% (n = 171) and 11.3% (n = 141) were acquired in Asia and the Americas, respectively (Table 3). A limited number of imported cases were acquired in Oceania (3.0%; n = 37). The highest concentration of cases acquired in Africa came from countries in West Africa (69.7%; n = 629); a substantial percentage of cases acquired in Asia came from the Indian subcontinent (52.6%; n = 90). From within the Americas, the majority of cases were acquired in Central America and the Caribbean (68.1%; n = 96), followed by South America (24.8%; n = 35) and Mexico (7.1%; n = 10). Information regarding region of acquisition was missing for 80 (6.4%) of the imported cases. Compared with 2001, the number of reported malaria cases acquired in Asia and Africa increased by 4.3% and 1.9%, respectively, and the number of cases acquired in the Americas decreased by 41.2%. In the United States, the five health departments reporting the highest number of malaria cases were New York City (n = 202), California (n = 197), Maryland (n = 101), Florida (n = 87), and Texas (n = 67) (Figure 2). Whereas the majority of these health departments reported an increase in cases compared with 2001, an overall decrease in cases occurred nationwide. This decrease probably represents year-to-year variation in malaria cases rather than a trend but could also have resulted from local changes in disease transmission, decreased travel to malaria-endemic regions, fluctuation in reporting to state and local health departments, or an increased use of effective antimalarial chemoprophylaxis. Interval Between Arrival and IllnessThe interval between date of arrival in the United States and onset of illness and the infecting Plasmodium species were known for 681 (51.1%) of the imported cases of malaria (Table 4). Symptoms began before arrival in the United States for 92 (13.5%) persons, whereas symptoms began after arrival in the United States for 589 (86.5%) of these patients. Clinical malaria developed within 1 month after arrival in 385 (79.9%) of the 482 P. falciparum cases and in 57 (36.8%) of the 155 P. vivax cases (Table 4). Only seven (1.0%) of the 681 persons became ill >1 year after returning to the United States. Imported Malaria CasesImported Malaria Among U.S. Military PersonnelIn 2002, a total of 33 cases of imported malaria were reported among U.S. military personnel. These cases were reported by state health departments. Of the 28 cases for whom information regarding chemoprophylaxis use was available, 19 (67.9%) patients were not using any chemoprophylaxis. Imported Malaria Among Civilians A total of 1,121 imported malaria cases were reported among civilians. Of these, 849 (75.7%) cases occurred among U.S. residents, and 272 (24.3%) cases occurred among residents of other countries (Table 5). Of the 849 imported malaria cases among U.S. civilians, 641 (75.5%) had been acquired in Africa, an increase of 1.1% from cases reported in 2001. Asia accounted for 89 (10.5%) cases of imported malaria among U.S. civilians, and travel to the Central American and Caribbean regions accounted for an additional 57 (6.7%) cases. Of the 272 imported cases among foreign civilians, the majority of cases were acquired in Africa (66.2%; n = 180). Antimalarial Chemoprophylaxis UseChemoprophylaxis Use Among U.S. Civilians Information concerning chemoprophylaxis use and travel area was known for 799 (94.1%) of the 849 U.S. civilians who had imported malaria. Of these 799 persons, 482 (60.3%) had not taken any chemoprophylaxis, and 136 (17.0%) had not taken a CDC-recommended drug for the area visited (9). Only 167 (20.9%) U.S. civilians had taken a CDC-recommended medication (9). Data for the specific drug taken were missing for the remaining 14 (1.8%) travelers. A total of 110 (65.9%) patients on CDC-recommended prophylaxis had reported taking mefloquine weekly; 30 (18.0%) had taken doxycycline daily; nine (5.4%) had taken atovaquone-proguanil daily; and six (3.6%) who had traveled only in areas where chloroquine-resistant malaria has not been documented had taken chloroquine weekly. Information on adherence to the drug regimen for these persons is presented in the following section. Twelve patients (7.2%) had taken combinations of drugs that included >1 CDC-recommended drug for the travel region. Of the 136 patients taking a nonrecommended drug, 67 (49.3%) reported taking chloroquine either alone or in combination with another ineffective drug during travel to an area where chloroquine resistance has been documented. Malaria Infection After Recommended Prophylaxis Use A total of 185 patients (i.e., 167 U.S. civilians, eight persons in the U.S. military, three foreign civilians, and seven persons whose information regarding their status was missing) experienced malaria after taking a recommended antimalarial drug for chemoprophylaxis. Information regarding infecting species was available for 158 (85.4%) patients taking a recommended antimalarial drug; the infecting species was undetermined for the remaining 27. Cases of P. vivax or P. ovale After Recommended Prophylaxis Use. Of the 185 patients who experienced malaria after recommended chemoprophylaxis use, 69 cases (37.3%) were caused by P. vivax and 13 (7.0%) by P. ovale. Twenty-two (26.8%) of these 82 patients were noncompliant with antimalarial chemoprophylaxis. A total of 41 (50.0%) cases of P. vivax or P. ovale occurred >45 days after arrival in the United States. These cases were consistent with relapsing infections and, thus, do not indicate primary prophylaxis failures. Information was insufficient, because of missing data regarding symptom onset or return date, to assess whether 28 cases were relapsing infections. Thirteen cases, 10 by P. vivax and three by P. ovale, occurred <45 days after the patient returned (n = 9) or before return (n = 4) to the United States. Six of the 13 patients were known to be noncompliant with their antimalarial chemoprophylaxis regimen, and four patients were not known to be noncompliant. The region of acquisition varied for the four patients who were not known to be noncompliant (one from East Africa, one from West Africa, one from Central Africa, and one from Asia). The remaining three patients reported compliance with an antimalarial chemoprophylaxis regimen. Of these three, two had traveled to Papua New Guinea and one to sub-Saharan Africa. Two of these patients reported taking mefloquine, and one reported using doxycycline. Blood samples for serum drug levels were not available for these three patients. The possible explanations for these cases include inappropriate dosing, noncompliance that was not reported, malabsorption of the drug or emerging parasite resistance. Cases of P. falciparum and P. malariae after Recommended Prophylaxis Use. The remaining 103 cases of malaria reported among persons who had taken a recommended antimalarial drug for chemoprophylaxis include 69 cases of P. falciparum, six cases of P. malariae, one case of mixed infection, and 27 cases in which the infecting species was unidentified. A total of 61 of the 69 P. falciparum cases among those who reported taking a recommended antimalarial drug were acquired in Africa, five in Asia, and three in Oceania. In 42 (60.9%) of these 69 cases, noncompliance with antimalarials was reported. In five (7.2%) of these 69 cases, patients reported compliance with antimalarial chemoprophylaxis. All five of these patients had traveled to Africa. Of the four who had traveled to West Africa, three had traveled to Ghana and one to Sierra Leone. Three had reported taking mefloquine, and two had reported taking atovaquone-proguanil for malaria chemoprophylaxis. A mefloquine blood level was available for one of the patients who had traveled to Ghana; this patient's mefloquine level was undetectable, thus indicating either noncompliance with the recommended regimen or complete malabsorption of the drug. Blood samples were not available for the remaining four patients who reported compliance with a recommended regimen. Twenty-two cases occurred of P. falciparum for which patient compliance was unknown. The majority of these cases were acquired in Africa (n = 19): 11 in West Africa, three in East Africa, two in Central Africa, and three in an unspecified African region. Three cases were acquired outside Africa: one in Indonesia and two in Papua New Guinea. Blood samples were not available for the 22 patients whose compliance status was unknown. Five of the six P. malariae cases among those who reported taking a recommended antimalarial drug were acquired in Africa. In three (50.0%) of these six cases, noncompliance with antimalarials was reported. One (16.7%) case reported compliance with a recommended chemoprophylaxis regimen using doxycycline. This patient traveled to southern Africa and became ill before returning to the United States. In the two remaining cases, patient compliance with prophylaxis was unknown and blood samples were not available; both had traveled in Africa. Purpose of TravelPurpose of travel to malaria-endemic areas was reported for 745 (87.8%) of the 849 U.S. civilians with imported malaria (Table 6). Of the U.S. civilians with malaria, the largest proportion (45.0%) were persons who had visited friends or relatives in malarious areas; the second and third highest proportion, 10.6% and 10.2%, had traveled to do missionary work and for tourism, respectively. Malaria During PregnancyA total of 32 cases of malaria were reported among pregnant women in 2002, representing 7.4% of cases among women. Twelve of the 32 (37.5%) were among U.S. civilians. Six of these twelve women had traveled to visit friends and relatives; seven had traveled in Africa, and five in Asia. A total of 28.1% of pregnant women and 28.7% of nonpregnant women reported taking malaria chemoprophylaxis. Malaria Acquired in the United StatesCongenital Malaria One case of congenital malaria was reported in 2002 and is described in the following case report:
Cryptic Malaria Three cases of cryptic malaria were reported in 2002 and are described in the following case reports:
These two cases from Northern Virginia were investigated by local public health officials and CDC, who concluded that the cases represented an outbreak of locally acquired mosquito-transmitted malaria. The investigation revealed that the patient aged 19 years often visited friends who lived directly across the street from the home of the patient aged 15 years. PCR was performed on blood from both patients, and it revealed that the infecting parasites were genotypically identical to each other, indicating a common source. Medical charts from two local hospitals were reviewed, and local physicians were contacted; however, no other cases of malaria were identified (10). Induced Malaria One case of induced malaria was reported in 2002 and is described in the following case report:
Deaths Attributed to MalariaEight deaths attributable to malaria were reported in 2002 and are described in the following case reports:
DiscussionA total of 1,337 cases of malaria were reported to CDC for 2002, representing a 3.3% decrease from the 1,383 cases reported for 2001. This change primarily resulted from a decrease in cases acquired in the Americas. Since 2000, CDC has routinely contacted state health departments to ask for outstanding malaria case reports from the previous reporting year or for a statement that reporting is complete. The decrease in cases in 2002, compared with 2001, probably is a result of expected variation in the number of cases, although other possibilities include decreased international travel, changing patterns of travel (e.g., decreased immigration from malarious areas), or an increased use of effective antimalarial chemoprophylaxis. One reason for conducting malaria surveillance is to monitor for prophylaxis failures that might indicate emergence of drug resistance; however, approximately 75% of imported malaria among U.S. civilians occurred among persons who were either not taking prophylaxis or were taking nonrecommended prophylaxis for the region to which they were traveling. Of the cases where appropriate prophylaxis was reported and for whom adequate information was available regarding species and onset of symptoms to indicate that the infection was a primary one rather than a relapse, the majority reported noncompliance with recommended regimen or had insufficient information to determine whether these cases represented problems with adherence while using correct antimalarial chemoprophylaxis, malabsorption of the antimalarial drug, or emerging drug resistance. Among patients who reported compliance with a recommended regimen, serum drug levels were only available for one patient. Therefore, differentiating among inaccurate reporting of compliance, malabsorption of the antimalarial drug, and emerging drug resistance is impossible. No conclusive evidence exists to indicate a single national or regional source of infection among this group of patients or the failure of a particular chemoprophylactic regimen. Health-care providers are encouraged to contact CDC rapidly whenever they suspect chemoprophylaxis failure, thus enabling measurement of serum drug levels of the antimalarial drugs in question. In 2001, to better evaluate chemoprophylaxis failures, CDC revised the NMSS case report form to facilitate collection of more thorough data regarding chemoprophylaxis. The revised form solicits more detailed information regarding the prescribed regimen, the degree of compliance with the regimen, and the reasons for noncompliance, if any. Data gathered from the responses will be useful in generating public health messages to improve use of antimalarial chemoprophylaxis and therefore decrease malaria-associated morbidity and mortality among U.S. civilians. The importance of taking correct precautions and chemoprophylaxis is underscored by the eight fatal cases of malaria that occurred in the United States in 2002. An earlier review of deaths attributed to malaria in the United States identified specific risk factors for fatal malaria, including failure to take recommended antimalarial chemoprophylaxis, refusal of or delay in seeking medical care, and misdiagnosis (11). The occurrence of 12 cases of malaria among pregnant U.S. civilians is also cause for concern. Malaria during pregnancy among nonimmune women is more likely to result in severe disease or contribute to an adverse outcome than malaria in nonpregnant women (12); the fetus might be adversely affected as well (13). Pregnant travelers should be counseled to avoid travel to malarious areas, if possible. If deferral of travel is impossible, pregnant women should be informed that the risks for malaria outweigh those associated with prophylaxis and that safe chemoprophylaxis regimens are available. Specific guidance for pregnant travelers is available from CDC's Internet site at http://www.cdc.gov/travel/mal_preg_pub.htm. Signs and symptoms of malaria are often nonspecific, but fever is usually present. Other symptoms include headache, chills, increased sweating, back pain, myalgia, diarrhea, nausea, vomiting, and cough. Prompt diagnosis requires that malaria be included in the differential diagnosis of illness in a febrile person with a history of travel to a malarious area. Clinicians should ask all febrile patients for a travel history, including when evaluating febrile illnesses among international visitors, immigrants, refugees, migrant laborers, and international travelers. Prompt treatment of suspected malaria is essential, because persons with P. falciparum infection are at risk for experiencing life-threatening complications soon after the onset of illness. Ideally, therapy for malaria should be initiated immediately after the diagnosis has been confirmed by a positive blood film. Treatment should be determined on the basis of the infecting Plasmodium species, the probable geographic origin of the parasite, the parasite density, and the patient's clinical status (14). If the diagnosis of malaria is suspected and cannot be confirmed, or if a diagnosis of malaria is confirmed but species determination is not possible, antimalarial treatment should be initiated that is effective against P. falciparum. Resistance of P. falciparum to chloroquine is worldwide, with the exception of a limited number of geographic regions (e.g., Central America). Therefore, therapy for presumed P. falciparum malaria should usually entail use of a drug effective against such resistant strains. Health-care providers should be familiar with prevention, recognition, and treatment of malaria and are encouraged to consult appropriate sources for malaria prevention and treatment recommendations (Table 7). Physicians seeking assistance with the diagnosis or treatment of patients with suspected or confirmed malaria should call CDC's National Center for Infectious Diseases, Division of Parasitic Diseases, at 770-488-7788 during regular business hours or CDC's Emergency Operations Center, at 770-488-7100 during evenings, weekends, and holidays (ask to page person on call for Malaria Branch). These resources are intended for use by health-care professionals only. Detailed recommendations for preventing malaria are available to the general public 24 hours/day from CDC by telephone at 877-394-8747 (toll-free voice information system) or 888-232-3299 (toll-free facsimile request line), or on the Internet at http://www.cdc.gov/travel/diseases.htm#malaria. In addition, CDC biannually publishes recommendations in Health Information for International Travel (commonly referred to as The Yellow Book) (10), which is available for purchase from the Public Health Foundation (telephone: 877-252-1200 or 301-645-7773); it is also available and updated more frequently on CDC's Internet site at http://www.cdc.gov/travel. CDC provides technical support for health-care providers in diagnosing malaria through DPDx, a program that enhances diagnosis of parasitic diseases throughout the world. It includes an Internet site, http://www.dpd.cdc.gov/DPDx/, that contains information regarding laboratory diagnosis, geographic distribution, clinical features, treatment, and life cycles of >100 different parasite species, including malaria parasites. The DPDx Internet site is also a portal for diagnostic assistance for health-care providers through telediagnosis. Digital images captured from diagnostic specimens can be submitted for diagnostic consultation through electronic mail. Because laboratories can transmit images to CDC and rapidly obtain answers to their inquiries, this system allows efficient diagnosis of difficult cases and rapid dissemination of information. Approximately 46 public health laboratories in 41 states, Puerto Rico, and Guam have, or are in the process of acquiring, the hardware to perform telediagnosis. AcknowledgmentsThe authors acknowledge the state, territorial, and local health departments; health-care providers; and laboratories for reporting this information to CDC. References
* To obtain confirmation diagnosis of blood films from questionable cases and to obtain appropriate treatment recommendations, contact either your state or local health department or CDC's National Center for Infectious Diseases, Division of Parasitic Diseases, Malaria Epidemiology Branch at 770-488-7788. Table 1 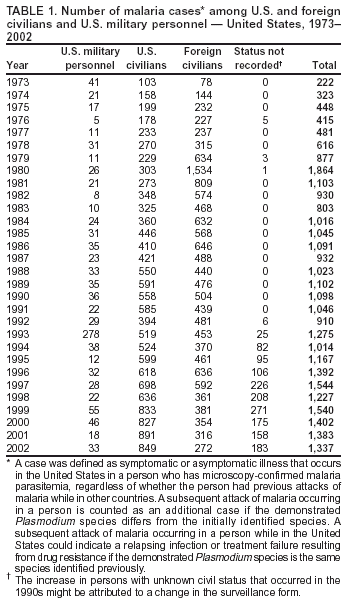 Return to top. Figure 1 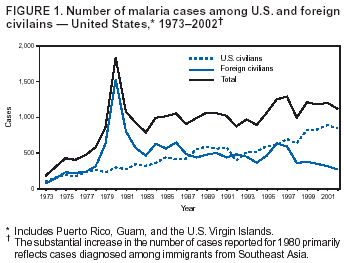 Return to top. Table 2 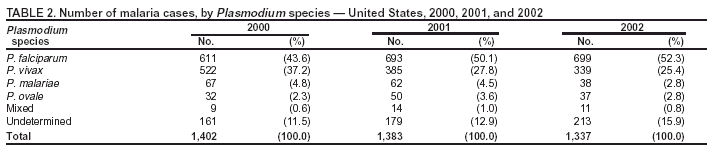 Return to top. Figure 2 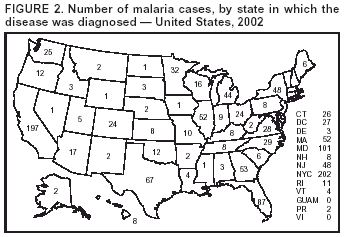 Return to top. Table 3 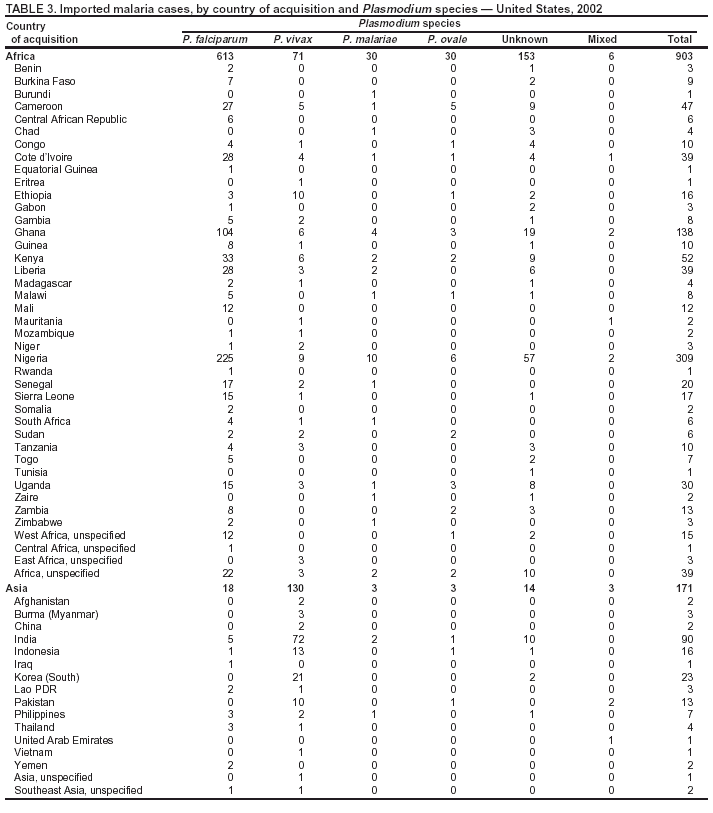 Return to top. Table 4 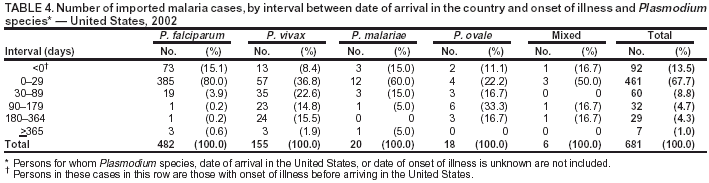 Return to top. Table 5 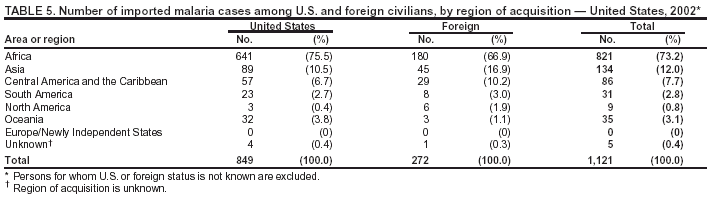 Return to top. Table 6 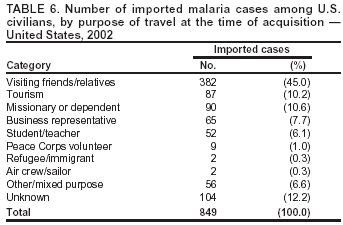 Return to top. Table 7 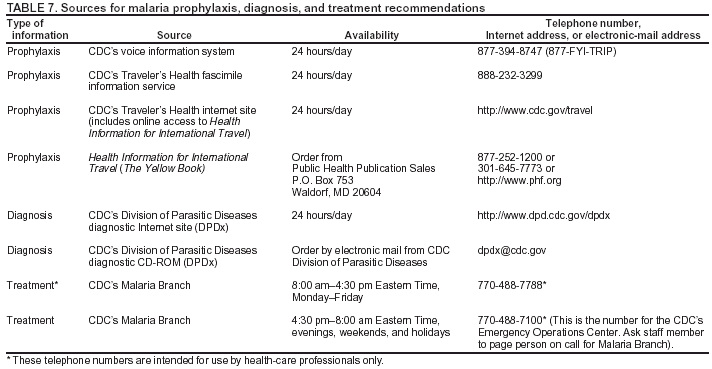 Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/19/2004 |
|||||||||
This page last reviewed 4/19/2004
|