 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Surveillance for Lyme Disease --- United States, 1992--1998Kathleen A. Orloski, D.V.M., M.S. AbstractProblem/Condition: Lyme disease is caused by infection with the spirochete Borrelia burgdorferi and is the most commonly reported vectorborne disease in the United States. Borrelia burgdorferi is transmitted to humans by infected Ixodes scapularis and I. pacificus ticks. Lyme disease is typically evidenced in its early stage by a characteristic rash (erythema migrans), accompanied by nonspecific symptoms (e.g., fever, malaise, fatigue, headache, myalgia, and arthralgia). Lyme disease can usually be treated successfully with standard antibiotic regimens. Reporting Period: 1992--1998. Description of System: Lyme disease surveillance data are reported to CDC through the National Electronic Telecommunication System for Surveillance, a computerized public health database for nationally notifiable diseases. During 1992--1998, data regarding reported cases of Lyme disease included county and state of residence, age, sex, and date of onset. Descriptive analyses were performed, and cumulative incidence by state, county, age group, and sex were calculated. Results: During 1992--1998, a total of 88,967 cases of Lyme disease was reported to CDC by 49 states and the District of Columbia, with the number of cases increasing from 9,896 in 1992 to 16,802 in 1998. A total of 92% of cases was reported from eight northeastern and mid-Atlantic states and two north-central states. Children aged 5--9 years and adults aged 45--54 years had the highest mean annual incidence. Interpretation: Lyme disease is a highly focal disease, with the majority of reported cases occurring in the northeastern and north-central United States. The number of reported cases of Lyme disease increased during 1992--1998. Geographic and seasonal patterns of disease correlate with the distribution and feeding habits of the vector ticks, I. scapularis and I. pacificus. Public Health Action: The results presented in this report will help clinicians evaluate the prior probability of Lyme disease and provide the framework for targeting human Lyme disease vaccine use and other prevention and treatment interventions. INTRODUCTIONLyme disease is caused by infection with the spirochete Borrelia burgdorferi and is the most commonly reported vectorborne disease in the United States (1), where the vectors are Ixodes scapularis and I. pacificus ticks. Lyme disease was first described in the United States in 1977 as Lyme arthritis (2), although earlier publications described cases of erythema migrans (3,4). Erythema migrans, also referred to as a bulls-eye rash, is the hallmark symptom of Lyme disease. In 1982, systematic surveillance for Lyme disease was initiated by CDC, with 11 states reporting 491 cases (5). During 1982--1988, the number of reported cases increased steadily, probably because of increased surveillance and a true increase in incidence (6,7). By 1985, a total of 2,748 cases was reported by 25 states, and by 1988, a total of 4,882 cases was reported by 43 states. In 1990, a standardized case definition was approved by the Council of State and Territorial Epidemiologists, and in 1991, that case definition was implemented nationwide. During 1989--1991, the number of reported cases increased slightly (Figure 1). Infection with B. burgdorferi is preventable by avoiding exposure to tick-infested habitats in Lyme disease-endemic areas. Recommended personal prevention behaviors include applying tick repellents and acaricides, wearing light-colored clothing to make ticks more visible, tucking pants into socks, and performing tick checks after coming in from the outdoors. These protective measures can be inconvenient to perform daily and are often underutilized (8,9). New strategies to decrease the risk for infection are being developed and evaluated for effectiveness and include human vaccines and host-targeted acaricides (e.g., self-application of acaricides by deer at feeding stations). The U.S. Food and Drug Administration has approved a vaccine for human use (LYMErix,™ Smith-Kline Beecham Pharmaceuticals) (10), and in June 1999, CDC published recommendations from the Advisory Committee on Immunization Practices for the use of that vaccine (11). Those recommendations state that vaccination for Lyme disease should be considered for persons aged 15--70 years who live in areas of moderate to high risk for Lyme disease and have frequent or prolonged contact with tick habitat. A risk map is included in the appendix to that report that categorizes risk by U.S. county as none, low, moderate, and high. Vaccination is not recommended for persons with treatment-resistant Lyme arthritis or pregnant women. This report includes the demographic characteristics and seasonal and geographic distribution of reported cases of Lyme disease that occurred during 1992--1998. These results provide a basis for evaluating the clinical probability of Lyme disease and for targeting prevention efforts, including the use of the recently approved Lyme disease vaccine. MATERIALS AND METHODSReported cases of Lyme disease were submitted electronically by state health departments to CDC through the National Electronic Telecommunications System for Surveillance (NETSS). For surveillance purposes, a case of Lyme disease is defined as an illness consisting of either a) physician-diagnosed erythema migrans >5 cm in diameter or b) at least one disseminated manifestation (e.g., musculoskeletal, neurologic, or cardiac) plus laboratory confirmation of infection (12). The recommended testing protocol for Lyme disease consists of two steps (12). The first step is testing with a highly sensitive enzyme immunoassay or immunofluorescence assay. If the first test is either equivocal or positive, a Western immunoblot test should be performed. If the first test is negative, no further testing is necessary. Cases of Lyme disease are reported through a combination of passive, active, and laboratory-based surveillance. Lyme disease reporting was enhanced through laboratory-based or active surveillance programs for at least a certain portion of Connecticut, Maryland, Massachusetts, Michigan, Minnesota, New Jersey, New York, Oregon, Rhode Island, West Virginia, and Wisconsin. These enhanced reporting programs were often not in place for the entire reporting period; for example, several states added laboratory-based surveillance for Lyme disease only in the last several years. Passive reporting is initiated when a health-care provider makes a diagnosis of Lyme disease and reports that case to the local public health office or directly to the state health department. The state health department determines if the case meets the case definition, including what laboratory tests will be used to confirm cases of disseminated Lyme disease. Reported cases meeting the case definition of Lyme disease are submitted electronically by state health departments to CDC through NETSS. Methods for conducting active surveillance vary, but usually require public health staff to regularly contact health-care providers for information regarding newly diagnosed cases of Lyme disease. In laboratory-based surveillance, diagnostic laboratories are required by law to report all positive Lyme disease test results to the health department. Because limited patient information is usually provided with the laboratory results, health department staff must follow up with health-care providers and collect the clinical information necessary to determine if these cases meet the case definition. For this report, data regarding reported cases included county and state of residence, age, sex, and date of illness onset. In addition, the earliest available date of the illness event was included, whether that date was of onset, diagnosis, or report. Selected reports also included clinical data, but these data were reported in such a low percentage of cases that they were not considered representative and were not included in the analysis. During 1992--1993, only aggregate case counts were available from Pennsylvania and, for 1992, from Oregon.* Population data from Guam were not included in any calculations of incidence. Descriptive analysis was performed by using Epi-Info (13) and Microsoft Excel® (14) computer programs. Reported cumulative incidence by state, county, age group, and sex were calculated by using 1990 census data. RESULTSDuring 1992--1998, a total of 88,967 cases of Lyme disease was reported by 49 states, the District of Columbia, and Guam (2 cases), for a crude mean annual incidence of 5.1 reported cases/100,000 persons/year. The number of reported cases increased 70%, from 9,909 in 1992 to 16,802 in 1998 (Figure 1). Ninety two percent of cases were reported by 10 states (Table). Over the 7-year period, crude annual incidence per 100,000 persons increased from 4.0 to 6.7. The 7-year mean annual reported incidence per 100,000 persons by state ranged from 0 to 67.9/100,000 persons (median: 0.6). A total of 6,752 cases (7.6%) was reported from 39 states with low or no known Lyme disease risk over the 7-year period. Information regarding county of residence was available for 85,382 cases (96.0%). Of the 3,143 counties in the United States, 1,646 (52.4%) reported at least 1 case during the 7-year period. The total number of cases reported by an individual county ranged from 1 (505 counties) to 7,882 (1 county) (median: 3 cases). The top 10% of counties (n = 165) reported 78,187 cases (91.6%) (Figure 2). The number of counties reporting at least 1 case of Lyme disease annually ranged from 617 to 710 (mean: 689; median: 689). The mean 7-year (5-year for Pennsylvania) annual reported incidence by counties reporting at least 1 case ranged from 0.02 to 1009.9/100,000 persons (median: 1.1). Within numerous endemic states, the distribution of reported cases was concentrated in a limited number of counties. In New York, 81.9% of cases with known county of residence were from 5 of 62 counties, and in Rhode Island, 69.4% of cases with known county of residence were reported from 1 of 5 counties. However, in Connecticut, the top two counties reported 45.9% of cases with known county of residence; the remainder of cases were distributed uniformly across the state. These trends remained somewhat consistent across reporting periods. Information regarding age was available for 86,425 reported cases (97.1%). The age distribution of reported cases was bimodal (Figure 3). The median age was 39.0 years (range: age <1--100 years), and the highest reported incidence occurred in children aged 5--9 years and adults aged 45--54 years. Information regarding sex was available for 85,540 cases (96.2%); of these, 44,386 cases (51.9%) occurred among males. Crude mean annual incidence was 4.8/100,000 males and 4.3/100,000 females. For children and adolescents aged 5--19 years, and adults aged >60 years, reported incidence was higher among males (Figure 3). For all other age groups, reported incidence was approximately equal among males and females. Month of disease onset was available for 64,423 (72.4%) reported cases. The majority of these cases had onset in June (23.6%), July (30.8%), or August (12.5%), although disease onset was reported to occur in all months of the year (Figure 4). February was the month of disease onset with the lowest number of reported cases (1.6%). DISCUSSIONDuring 1992--1998, the annual number of reported cases of Lyme disease increased, with the majority of cases reported from the northeast and north-central regions of the United States. The majority of cases reported onset in the spring and summer, and children aged 5--9 years and adults aged 45--54 years had the highest reported incidences. These findings are important for determining the underlying risk for Lyme disease during patient evaluation and when targeting prevention efforts, including the use of vaccine. The increase in reported cases is probably a result of both a true increase in incidence within known endemic areas as well as more complete reporting as a result of enhanced Lyme disease surveillance in endemic states (15,16). Past surveillance efforts have reported an increase in the incidence of cases concurrent with the geographic spread of I. scapularis (7). Within endemic areas, increases in tick abundance have been observed (17--19). However, surveillance capabilities and public awareness of Lyme disease have increased as well. During the reporting period, the total number of counties reporting at least one case of Lyme disease annually did not increase. Approximately the same counties appear in the top 10% each year, but with each county reporting substantially more cases over time. These data indicate that the increase in reported cases has occurred primarily within known Lyme disease-endemic areas. As with a majority of diseases reported through a passive surveillance system, Lyme disease is underreported. Studies in Connecticut and Maryland estimated 7--12 unreported cases for each reported case (20,21). Additionally, the case definition has limitations of sensitivity and specificity. Although the case definition was written to be highly sensitive, some unknown proportion of persons with Lyme disease will not meet the case definition (e.g., a person with an erythema migrans <5 cm in diameter). Conversely, misdiagnosis of Lyme disease is known to occur (22--26), yet some of these cases are undoubtedly reported as Lyme disease and meet the case definition. Despite these problems, Lyme disease surveillance provides a useful measure of trends in incidence and geographic distribution of Lyme disease (19). Lyme disease has a highly focal distribution within the United States. The top 10% of counties reported approximately 92% of cases for which county of residence was known. These counties are predominantly located in eight northeastern states (Connecticut, Delaware, Maryland, Massachusetts, New Jersey, New York, Pennsylvania, and Rhode Island) and two north-central states (Minnesota and Wisconsin). This focal distribution of human cases correlates well with the distribution, density, and infection prevalence of I. scapularis in the northeastern and north-central United States (17,27--29). Cases are reported by county of residence, which for the purposes of this analysis, is assumed to be the county of exposure. This assumption is reasonable for northeastern states because persons in these states are usually exposed to infected ticks periresidentially (16,27,30,31). However, a study of Lyme disease cases in Wisconsin indicated that persons were often exposed to infected ticks outside their county of residence (29). The majority of reported cases had onsets of disease in June, July, or August. This is consistent with the results of other epidemiologic studies (16,32--35) and corresponds with the seasonal feeding activity of nymphal I. scapularis in the northeastern United States (36). In addition, June, July, and August are the months when humans most commonly engage in outdoor activities. Researchers believe that a majority of human cases result from nymphal tick attachment. Because the attached nymph is approximately the size of a poppy seed, it might not be noticed and, therefore, not removed before disease transmission occurs (36). The reporting of cases with later disseminated stages of Lyme disease is not expected to indicate strong seasonality, which could explain why certain cases are reported with onset during the winter months; others could have resulted from exposure to adult ticks, which feed from fall to early spring. Reported incidence was highest for children aged 5--9 years and adults aged 45--54 years. The reported incidence for males was higher than for females, notably in the age groups of 5--19 and >60 years. These findings could be a result of a true increase in risk associated with increased exposure to infected ticks, to decreased use of personal protective measures, or a result of reporting bias. Because the majority of Lyme disease surveillance data are collected passively, reported cases might not be representative of all cases of Lyme disease. Risk of Lyme disease increases with increasing exposure to wooded, brushy, or overgrown grassy areas in endemic regions (16,30,31,34,37) Less than 8% of cases were reported from states with low or no known risk for Lyme disease. Certain cases were acquired either outside the United States or within the United States but outside the state of residence (data not indicated). Others might be sporadic cases from states with overall low endemicity (e.g., California, Illinois, Maine, or Michigan), and a limited number of cases are unlikely to be cases of Lyme disease. Borrelia burgdorferi-infected Ixodes species other than I. scapularis or I. pacificus do occur in the United States, but are not known to transmit infection to humans (38--40). I. pacificus ticks are found in certain western states, although I. pacificus ticks infected with B. burgdorferi have been confirmed by isolation from ticks only in California and Oregon where infection rates range from 4% to 13.6% (41,42). Three cases in California residents have been confirmed by culture of B. burgdorferi from skin biopsies of erythema migrans lesions (CDC unpublished data, 1992). I. scapularis are found in the south-central and southeastern United States, but they infrequently bite humans and <3% are infected with B. burgdorferi (43). A U.S. map indicating risk for Lyme disease by county, which takes into account geographic variation in infection prevalence, has recently been published (11). To date, culture-confirmed Lyme disease cases in patients whose only exposure was in the south-central or southeastern United States are unknown. A skin rash resembling erythema migrans has been associated with bites by Amblyomma americanum, the Lone-Star tick, but apparently, the rash is not the result of infection with B. burgdorferi (44,45). Patients with such erythematous rashes might be mistakenly reported as having Lyme disease (46). A. americanum ticks are primarily found in the south-central and southeastern United States and are not a competent vector of B. burgdorferi (47,48). CONCLUSIONOngoing prevention and educational programs in endemic areas have stressed the use of personal protective measures. These measures might not be used extensively (8,9), and their effectiveness in preventing Lyme disease has not been demonstrated conclusively. Other prevention strategies attempt to reduce the density of I. scapularis in the environment and include deer exclusion or removal, application of acaricides or desiccants to vegetation, landscape management (e.g., removal of leaf litter), host-targeted acaricides, and the use of vaccine. To make the most efficient use of limited resources, prevention strategies should consider the geographic and temporal distribution of Lyme disease risk and appropriately target communities at moderate and higher risk. Studies regarding the inappropriate use of serologic tests and the overdiagnosis and inappropriate treatment of Lyme disease (25,26,49,50) provide evidence that physicians should consider the prior probability of disease when evaluating a patient for Lyme disease (51). Prior probability is determined in part by the underlying risk for Lyme disease in the community or region of exposure and the opportunity for exposure to infected ticks (11,51). A positive test result in a person with a low prior probability of having Lyme disease is probably a false positive test result (51). In nonendemic areas, public health programs can benefit by focusing on educational messages regarding the limited risk for acquiring Lyme disease; this effort could relieve public anxiety and reduce the occurrence of inappropriate testing and treatment of Lyme disease. In the future, Lyme disease surveillance, enhanced by laboratory reporting and expanded active surveillance, will be important in evaluating temporal and geographic trends and should be used to monitor the effectiveness of preventive interventions. References
* Cases and base population from Pennsylvania were excluded when calculating mean annual incidence by age group and sex. Cases and base population data from Oregon were included, but data for age, sex, and county of residence for 1992 were not available. Table 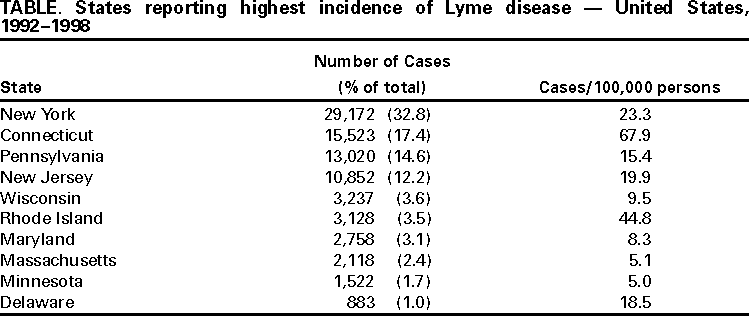 Return to top. Figure 1 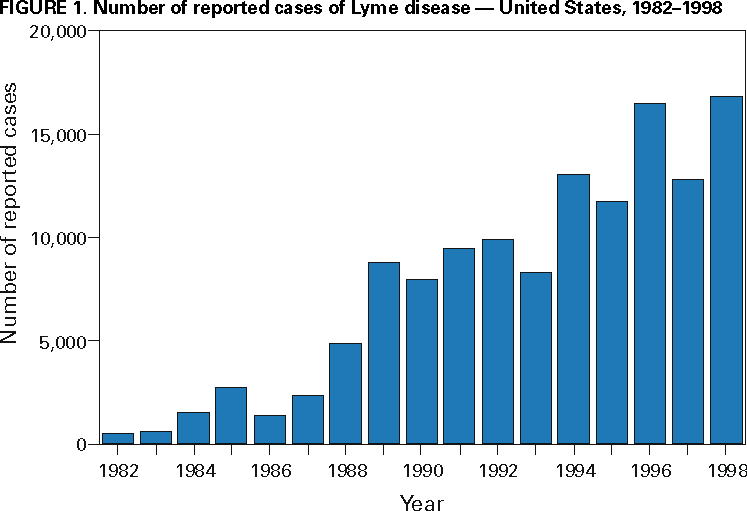 Return to top. Figure 2 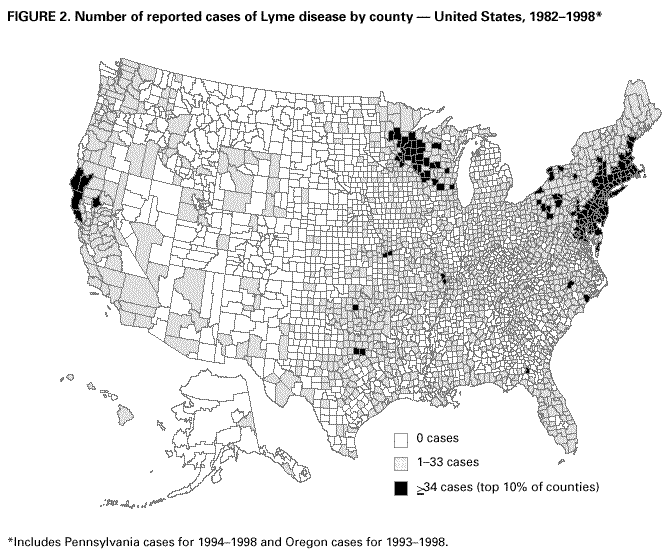 Return to top. Figure 3 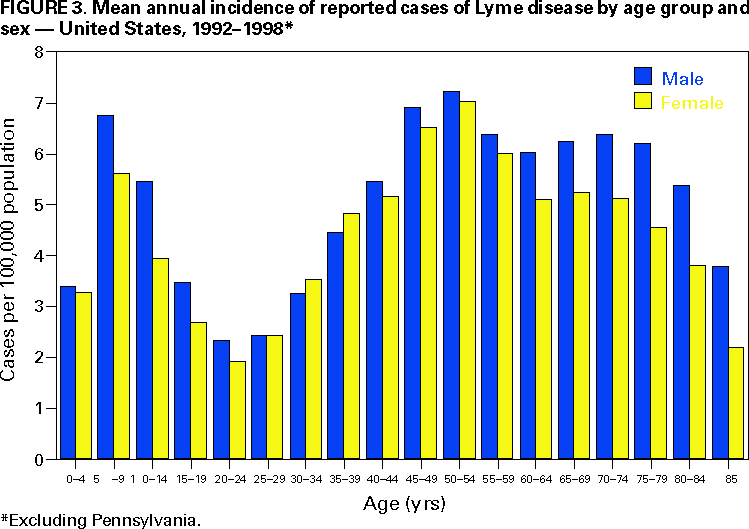 Return to top. Figure 4 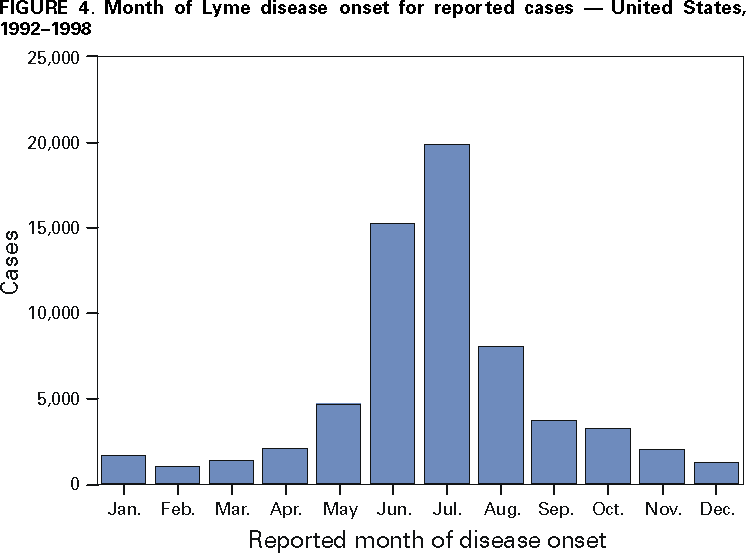 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/25/2000 |
|||||||||
This page last reviewed 5/2/01
|