 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Appendix ARegulatory Framework for Disinfectants and SterilantsWhen using the guidance provided in this report regarding use of liquid chemical disinfectants and sterilants, dental health-care personnel (DHCP) should be aware of federal laws and regulations that govern the sale, distribution, and use of these products. In particular, DHCPs should know what requirements pertain to them when such products are used. Finally, DHCP should understand the relative roles of the U.S. Environmental Protection Agency (EPA), the U.S. Food and Drug Administration (FDA), the Occupational Safety and Health Administration (OSHA) and CDC. The choice of specific cleaning or disinfecting agents is largely a matter of judgment, guided by product label claims and instructions and government regulations. A single liquid chemical germicide might not satisfy all disinfection requirements in a given dental practice or facility. Realistic use of liquid chemical germicides depends on consideration of multiple factors, including the degree of microbial killing required; the nature and composition of the surface, item, or device to be treated; and the cost, safety, and ease of use of the available agents. Selecting one appropriate product with a higher degree of potency to cover all situations might be more convenient. In the United States, liquid chemical germicides (disinfectants) are regulated by EPA and FDA (A-1--A-3). In health-care settings, EPA regulates disinfectants that are used on environmental surfaces (housekeeping and clinical contact surfaces), and FDA regulates liquid chemical sterilants/high-level disinfectants (e.g., glutaraldehyde, hydrogen peroxide, and peracetic acid) used on critical and semicritical patient-care devices. Disinfectants intended for use on clinical contact surfaces (e.g., light handles, radiographic-ray heads, or drawer knobs) or housekeeping surfaces (e.g., floors, walls, or sinks) are regulated in interstate commerce by the Antimicrobials Division, Office of Pesticide Programs, EPA, under the authority of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) of 1947, as amended in 1996 (A-4). Under FIFRA, any substance or mixture of substances intended to prevent, destroy, repel, or mitigate any pest, including microorganisms but excluding those in or on living man or animals, must be registered before sale or distribution. To obtain a registration, a manufacturer must submit specific data regarding the safety and the effectiveness of each product. EPA requires manufacturers to test formulations by using accepted methods for microbicidal activity, stability, and toxicity to animals and humans. Manufacturers submit these data to EPA with proposed labeling. If EPA concludes a product may be used without causing unreasonable adverse effects, the product and its labeling are given an EPA registration number, and the manufacturer may then sell and distribute the product in the United States. FIFRA requires users of products to follow the labeling directions on each product explicitly. The following statement appears on all EPA-registered product labels under the Directions for Use heading: "It is a violation of federal law to use this product inconsistent with its labeling." This means that DHCP must follow the safety precautions and use directions on the labeling of each registered product. Not following the specified dilution, contact time, method of application, or any other condition of use is considered misuse of the product. FDA, under the authority of the 1976 Medical Devices Amendment to the Food, Drug, and Cosmetic Act, regulates chemical germicides if they are advertised and marketed for use on specific medical devices (e.g., dental unit waterline or flexible endoscope). A liquid chemical germicide marketed for use on a specific device is considered, for regulatory purposes, a medical device itself when used to disinfect that specific medical device. Also, this FDA regulatory authority over a particular instrument or device dictates that the manufacturer is obligated to provide the user with adequate instructions for the safe and effective use of that device. These instructions must include methods to clean and disinfect or sterilize the item if it is to be marketed as a reusable medical device. OSHA develops workplace standards to help ensure safe and healthful working conditions in places of employment. OSHA is authorized under Pub. L. 95-251, and as amended, to enforce these workplace standards. In 1991, OSHA published Occupational Exposure to Bloodborne Pathogens; final rule [29 CFR Part 1910.1030] (A-5). This standard is designed to help prevent occupational exposures to blood or other potentially infectious substances. Under this standard, OSHA has interpreted that, to decontaminate contaminated work surfaces, either an EPA-registered hospital tuberculocidal disinfectant or an EPA-registered hospital disinfectant labeled as effective against human immunodeficiency virus (HIV) and hepatitis B virus (HBV) is appropriate. Hospital disinfectants with such HIV and HBV claims can be used, provided surfaces are not contaminated with agents or concentration of agents for which higher level (i.e., intermediate-level) disinfection is recommended. In addition, as with all disinfectants, effectiveness is governed by strict adherence to the label instructions for intended use of the product. CDC is not a regulatory agency and does not test, evaluate, or otherwise recommend specific brand-name products of chemical germicides. This report is intended to provide overall guidance for providers to select general classifications of products based on certain infection-control principles. In this report, CDC provides guidance to practitioners regarding appropriate application of EPA- and FDA-registered liquid chemical disinfectants and sterilants in dental health-care settings. CDC recommends disinfecting environmental surfaces or sterilizing or disinfecting medical equipment, and DHCP should use products approved by EPA and FDA unless no such products are available for use against certain microorganisms or sites. However, if no registered or approved products are available for a specific pathogen or use situation, DHCP are advised to follow the specific guidance regarding unregistered or unapproved (e.g., off-label) uses for various chemical germicides. For example, no antimicrobial products are registered for use specifically against certain emerging pathogens (e.g., Norwalk virus), potential terrorism agents (e.g., variola major or Yersinia pestis), or Creutzfeldt-Jakob disease agents. One point of clarification is the difference in how EPA and FDA classify disinfectants. FDA adopted the same basic terminology and classification scheme as CDC to categorize medical devices (i.e., critical, semicritical, and noncritical) and to define antimicrobial potency for processing surfaces (i.e., sterilization, and high-, intermediate- and low-level disinfection) (A-6). EPA registers environmental surface disinfectants based on the manufacturer's microbiological activity claims when registering its disinfectant. This difference has led to confusion on the part of users because the EPA does not use the terms intermediate- and low-level disinfectants as used in CDC guidelines. CDC designates any EPA-registered hospital disinfectant without a tuberculocidal claim as a low-level disinfectant and any EPA-registered hospital disinfectant with a tuberculocidal claim as an intermediate-level disinfectant. To understand this comparison, one needs to know how EPA registers disinfectants. First, to be labeled as an EPA hospital disinfectant, the product must pass Association of Official Analytical Chemists (AOAC) effectiveness tests against three target organisms: Salmonella choleraesuis for effectiveness against gram-negative bacteria; Staphylococcus aureus for effectiveness against gram-positive bacteria; and Pseudomonas aeruginosa for effectiveness against a primarily nosocomial pathogen. Substantiated label claims of effectiveness of a disinfectant against specific microorganisms other than the test microorganisms are permitted, but not required, provided that the test microorganisms are likely to be present in or on the recommended use areas and surfaces. Therefore, manufacturers might also test specifically against organisms of known concern in health-care practices (e.g., HIV, HBV, hepatitis C virus [HCV], and herpes) although it is considered likely that any product satisfying AOAC tests for hospital disinfectant designation will also be effective against these relatively fragile organisms when the product is used as directed by the manufacturer. Potency against Mycobacterium tuberculosis has been recognized as a substantial benchmark. However, the tuberculocidal claim is used only as a benchmark to measure germicidal potency. Tuberculosis is not transmitted via environmental surfaces but rather by the airborne route. Accordingly, use of such products on environmental surfaces plays no role in preventing the spread of tuberculosis. However, because mycobacteria have among the highest intrinsic levels of resistance among the vegetative bacteria, viruses, and fungi, any germicide with a tuberculocidal claim on the label is considered capable of inactivating a broad spectrum of pathogens, including such less-resistant organisms as bloodborne pathogens (e.g., HBV, HCV, and HIV). It is this broad-spectrum capability, rather than the product's specific potency against mycobacteria, that is the basis for protocols and regulations dictating use of tuberculocidal chemicals for surface disinfection. EPA also lists disinfectant products according to their labeled use against these organisms of interest as follows:
Microorganisms vary in their resistance to disinfection and sterilization, enabling CDC's designation of disinfectants as high-, intermediate-, and low-level, when compared with EPA's designated organism spectrum (Figure). However, exceptions to this general guide exist, and manufacturer's label claims and instructions should always be followed. ReferencesA-1. Food and Drug Administration (FDA) and US Environmental Protection Agency (EPA). Memorandum of understanding between the FDA and EPA: notice regarding matters of mutual responsibility---regulation of liquid chemical germicides intended for use on medical devices. Rockville, MD: US Department of Health and Human Services, Public Health Service, Food and Drug Administration, US Environmental Protection Agency, 1993. A-2. Food and Drug Administration (FDA). Interim measures for registration of antimicrobial products/liquid chemical germicides with medical device use claims under the memorandum of understanding between EPA and FDA. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, 1994. A-3. Food and Drug Administration. Guidance for industry and FDA reviewers: content and format of premarket notification [510(k)] submissions for liquid chemical sterilants/high level disinfectants. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, 2000. Available at http://www.fda.gov/cdrh/ode/397.pdf. A-4. US Environmental Protection Agency. 40 CFR Parts 152, 156, and 158. Exemption of certain pesticide substances from federal insecticide, fungicide, and rodenticide act requirements. Amended 1996. Federal Register 1996;61:8876--9. A-5. US Department of Labor, Occupational Safety and Health Administration. 29 CFR Part 1910.1030. Occupational exposure to bloodborne pathogens; needlesticks and other sharps injuries; final rule. Federal Register 2001;66:5317--25. As amended from and includes 29 CFR Part 1910.1030. Occupational exposure to bloodborne pathogens; final rule. Federal Register 1991;56:64174--82. Available at http://www. osha.gov/SLTC/dentistry/index.html. A-6. Spaulding EH. Role of chemical disinfection in preventing nosocomial infections. In: Proceedings of the International Conference on Nosocomial Infections, 1970. Brachman PS, Eickhoff TC, eds. Chicago, IL: American Hospital Association, 1971:247--54. Figure 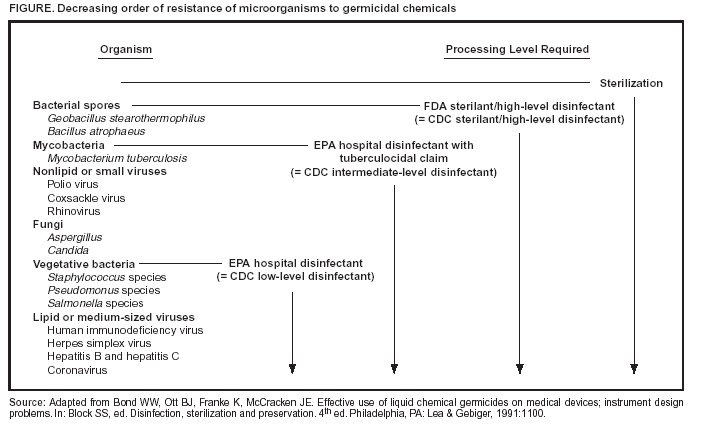 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/9/2003 |
|||||||||
This page last reviewed 12/9/2003
|