 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Two Simultaneous Outbreaks of Multidrug-Resistant Tuberculosis --- Federated States of Micronesia, 2007--2009In July 2008, CDC responded to a request from the Federated States of Micronesia (FSM) to investigate the first documented cases of multidrug-resistant tuberculosis (MDR TB) in Chuuk State. Compared with drug-susceptible TB disease, MDR TB is resistant to at least isoniazid and rifampin, the two most effective TB medications, making treatment more difficult and outcomes more likely fatal (1). Second-line TB drugs* for treating MDR TB were not available in FSM, and during December 2007--June 2008 four patients with MDR TB had died, including a child aged 2 years. This report describes the investigation by the World Health Organization (WHO) and CDC, which initially identified five confirmed cases in two distinct clusters, characterized by two distinct geographic locations, genotypes, and drug-susceptibility patterns. Extensive transmission has occurred among household contacts; 16 (8%) of the 205 contacts identified have confirmed or suspected MDR TB disease, and 124 (60%) have latent TB infection. Among 21 confirmed and suspected cases of MDR TB identified as of March 13, 2009, 10 have been in persons aged <15 years. With the death of a child aged 4 years in November 2008, a total of five persons have died of MDR TB. Multiple U.S. government agencies and other organizations are assisting local health authorities with resources to procure second-line TB drugs, ensure directly observed therapy (DOT), and identify and evaluate contacts. These simultaneous and continuing outbreaks demonstrate how a lack of basic TB control activities can allow the emergence and spread of drug-resistant TB. FSM comprises four states and more than 600 islands spread across 1 million square miles in the western Pacific Ocean. Half of the population of 108,000 lives in Chuuk, the largest state (2). TB is endemic in Chuuk, where 70 cases of TB were recorded in 2007. The 2007 incidence rate (127 TB cases per 100,000) is 29 times higher than the 2007 U.S. rate (3). Limited transportation hinders access to the only hospital in Chuuk, which provides chest radiography and smear microscopy services to help diagnose TB. Culture confirmation, drug-susceptibility testing, and genotyping were not available routinely for TB cases in FSM until January 2006, when referral laboratories in Hawaii and California began to offer these services. Before 2008, the state's geography, combined with limited TB program staffing, precluded active case-finding via routine contact investigations or the administration of DOT, a cornerstone of TB treatment that improves completion of therapy and prevents the emergence of drug resistance. Before July 2008, TB patients were identified as they showed signs or symptoms of TB disease at the local clinic or hospital; all received self-administered therapy. FSM's National TB Program has an annual budget of $170,000, and second-line drugs for treating MDR TB were not available because of funding constraints. In June 2007, pulmonary TB was diagnosed in a Chuuk resident aged 37 years. Sputum-smear microscopy detected acid-fast bacilli, and a chest radiograph showed lung cavitation, both indicators of contagiousness. In November 2007, drug-susceptibility test results confirmed multidrug resistance. The patient did not have access to second-line drugs and died. During December 2007--June 2008, four additional patients with MDR TB disease came to the local clinic or hospital. None of the four patients were treated with second-line drugs; three died, including a child aged 2 years. In May 2008, FSM authorities requested CDC assistance because of the 80% fatality rate and evidence of recent MDR TB transmission. A confirmed case was defined as laboratory-confirmed MDR TB disease in a Chuuk resident during January 2006--June 2008. A suspected case of MDR TB disease was defined as exposure (based on intensity and duration of contact) to a patient with confirmed TB and clinical findings of TB disease (i.e., laboratory confirmation pending). Patients (or proxies for deceased patients) were interviewed and laboratory and medical records reviewed. The July 2008 investigation focused on the initial five confirmed MDR TB cases. All patients were born in Chuuk; their median age was 16 years (range: 2--37 years), and four were female. None of the patients had a history of TB disease or treatment with TB drugs. All five patients had pulmonary TB, two with cavitation on chest radiograph and hemoptysis. None of the four patients who died before the investigation had been tested for human immunodeficiency virus (HIV) infection; the surviving patient had a negative HIV test result in July 2008. Two distinct clusters, associated with two different villages, were identified based on genotypes and drug-susceptibility patterns (Figure). In the first cluster of three cases, Mycobacterium tuberculosis isolates had a matching genotype and resistance to five drugs: isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin. This drug-susceptibility pattern had not been seen in Chuuk since routine drug-susceptibility testing became available in January 2006. The index patient in this cluster had worked during 1987--2000 in the garment industry in the Commonwealth of the Northern Mariana Islands (Saipan), which employs migrants from Southeast Asian countries (4) where MDR TB is common (5). In the second cluster of two cases, the isolates had a matching genotype and resistance to three drugs: isoniazid, rifampin, and ethionamide. The two patients were cousins whose extended family included five persons who previously had TB disease with isolates genotypically matched to those of the outbreak patients. However, the earlier isolates had resistance to isoniazid and ethionamide only. These five previous cases of non-MDR TB were diagnosed from January 2006, when genotyping became routinely available in Chuuk, to October 2007. Household caregivers reported that the five patients had self-administered therapy inconsistently or incompletely. During investigations of the five initial cases of MDR TB disease, a standardized clinical examination, chest radiography, and tuberculin skin testing were used to evaluate contacts for TB disease and latent TB infection (6). The 205 named contacts, of whom 163 (80%) were household members and 42 (20%) were health-care workers, had a median age of 20 years (range: 4 months--62 years), and 117 (57%) were female. During July 1, 2008--March 13, 2009, three additional MDR TB cases were confirmed among household members (Figure), including a household contact aged 4 years who in November 2008 died of meningitis later confirmed to be caused by MDR TB. Based on history of household exposure to a patient with confirmed TB and clinical findings (e.g., chest radiography consistent with TB disease), 13 other suspected cases of MDR TB disease were identified; all 13 patients began treatment based on the drug-susceptibility results of the respective source case (Table). Although fewer than one third of the contacts were aged <15 years (60 of 205), they accounted for more than half of the suspected and confirmed MDR TB cases since July 2008 (nine of 16). Latent TB infection was diagnosed in 124 (60%) contacts, although many of the adults were probably infected before this documented emergence of MDR TB in Chuuk. Among contacts aged <15 years, 20 (33%) of 60 had latent TB infection. All household contacts with latent TB infection have begun receiving second-line drugs based on the drug-susceptibility results of the respective source case (1,7). Multiple agencies have joined FSM in responding to the MDR TB outbreaks: the U.S. departments of Interior, Health and Human Services, State, and Defense; WHO; the Secretariat of the Pacific Community; and the Commonwealth of the Northern Mariana Islands Department of Public Health. Recommendations based on U.S. guidelines (8) and the International Standards for TB Care (9) have resulted in the following actions: 1) a consistent supply of fluoroquinolones, aminoglycosides, and other second-line drugs was procured; 2) Chuuk State Hospital added a separate ward for inpatient treatment of patients with TB; 3) TB program staff members received on-site training on providing DOT and conducting contact investigations; 4) nine new outreach workers were hired to administer DOT, and three vehicles were acquired to help workers investigate contacts; 5) the hospital laboratory was equipped for processing specimens for smear microscopy daily and shipping specimens for culture and drug-susceptibility testing weekly. For 2008, the improved case-detection capacity increased the recorded TB incidence to 204 cases per 100,000 persons. The Chuuk TB program is consulting with U.S. MDR TB experts by telephone and e-mail for assistance with complex treatment decisions, and implementing measures to prevent the selection of drug-resistant strains and reduce all TB transmission. Reported by: D Fred, MB, Chuuk TB Control Program; M Ekiek, MB, Federated States of Micronesia TB Control Program. B Pavlin, MD, World Health Organization. R Brostrom, MD, Commonwealth of the Northern Mariana Islands Dept of Public Health. M Haddad, MSN, S Bamrah, MD, A Heetderks, MPH, Div of TB Elimination, National Center for Viral Hepatitis, HIV/AIDS, STD, and TB Prevention; M Desai, MD, R Song, MD, EIS officers, CDC. Editorial Note:These two clusters of MDR TB represent two distinct outbreaks and illustrate two mechanisms for the emergence of drug resistance. In the first outbreak, the index patient had not been treated previously for TB and probably became infected with a MDR TB strain before returning to Chuuk in 2000 from Saipan; this case illustrates primary (i.e., initial) drug resistance. In the second outbreak, lack of DOT for the five family members with TB disease initially resistant to only isoniazid and ethionamide probably led to secondary (i.e., acquired) rifampin resistance. At least one of these five previous patients thus acquired multidrug resistance and transmitted MDR TB to the index case in the second outbreak. The emergence and transmission of MDR TB in these outbreaks were caused by the inability to follow standard TB control practices or to provide appropriate drugs. The findings also highlight the vulnerability of pediatric contacts and the challenges of diagnosing and treating MDR TB in resource-limited settings. Laboratory capacity and access to second-line TB drugs are fundamental to controlling MDR TB (1), and finding and curing all persons with TB is critical for interrupting transmission (8). Contact investigations enable active case-finding and early identification of recently infected contacts at highest risk for developing TB disease. Infection control practices (e.g., isolating contagious patients initially during treatment and wearing appropriate personal protective equipment) can prevent transmission of susceptible and drug-resistant TB, and are particularly important in congregate settings such as clinics, hospitals, and prisons. Uniform DOT for patients with TB disease prevents acquired drug resistance (5) and, where feasible, DOT should be offered for contacts with latent TB infection as well. The measures implemented in response to the MDR TB outbreaks in Chuuk have reflected all five aspects of the WHO global response plan for drug-resistant TB (10), which calls for augmenting the public health infrastructure to control TB, strengthening laboratory services for early diagnosis, improving surveillance to better understand drug resistance, implementing infection control to prevent transmission, and enhancing management of drug-resistant TB cases to reach the Global Plan to Stop TB 2006--2015 goals.† Tangible progress in treating and preventing the spread of TB has been made in Chuuk as recommendations from the investigation have been implemented. In 2008, an estimated 500,000 persons in the world developed MDR TB, largely as a result of inadequate TB control activities (5). In many countries where TB is endemic, ongoing transmission of multiple strains of MDR TB probably will be discovered as access to laboratory services improves (10). The challenge of primary drug resistance is likely to be exacerbated further by the increasing numbers of migratory and displaced populations (4). Many developing countries provide free first-line TB drugs through TB control programs. However, effective and sustainable mechanisms for access to expensive second-line TB drugs are needed for timely treatment of patients with drug-resistant TB. The multiagency response to the MDR TB outbreaks in Chuuk is a good example of the coordinated efforts that are needed to control MDR TB in many developing countries. As in Chuuk, a concerted focus on improving access to enhanced laboratory services and second-line TB drugs, and building local capacity for finding, diagnosing, and curing all forms of TB is necessary to address the global threat of MDR TB. Acknowledgments The findings in this report are based, in part, on contributions by M Kawamura, MD, Francis J. Curry Regional Treatment and Medical Consultation Center; M Bankowski, PhD, Diagnostic Laboratory Svcs; E Desmond, PhD, California Dept of Health Svcs Microbial Diseases Laboratory; R Wada, MPH, US Dept of Interior Office of Insular Affairs; C Wasem, MN, and J Walmsley, US Dept of Health and Human Svcs Office of the Regional Health Administrator, Region IX; M Hughes, US Dept of State; Capt J Parrish, MD, US Navy; USNS Mercy--Pacific Partnership 2008 mission; D Hamilton, MD, Office of Workforce and Career Development; and A Buff, MD, T Navin, MD, S Mase, MD, K Ijaz, MD, and J Jereb, MD, Div of TB Elimination, National Center for Viral Hepatitis, HIV/AIDS, STD, and TB Prevention, CDC. References
* Second-line TB drugs include aminoglycosides (e.g., amikacin, capreomycin, kanamycin), fluoroquinolones (e.g., ciprofloxacin, levofloxacin, and moxifloxacin), ethionamide, cycloserine, and para-aminosalicylic acid (PAS), among others. † Available at http://www.stoptb.org/globalplan. Table 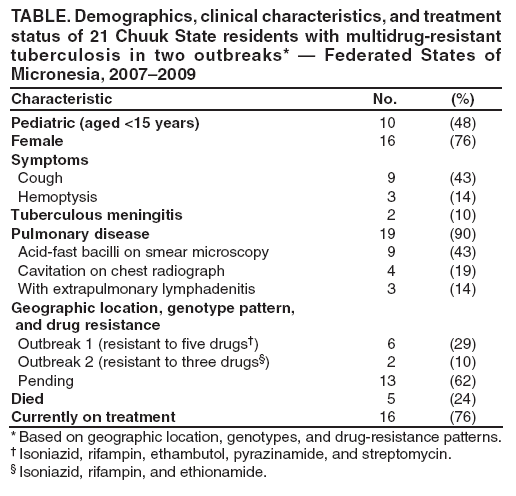 Return to top. Figure 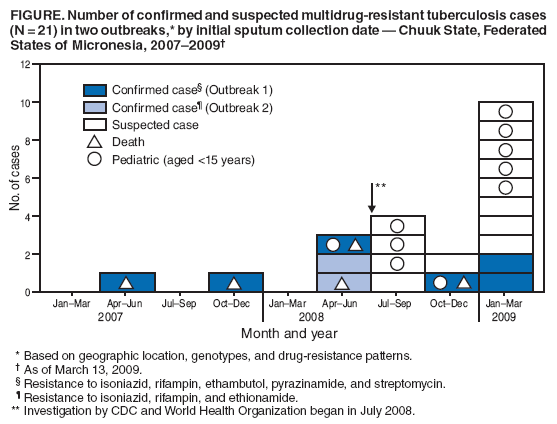 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 3/19/2009 |
|||||||||
|