 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Delayed Onset and Diminished Magnitude of Rotavirus Activity --- United States, November 2007--May 2008Rotavirus is the leading cause of severe acute gastroenteritis among infants and young children, accounting for an estimated 527,000 deaths among children aged <5 years worldwide in 2004 (1,2). In the United States, rotavirus causes few deaths (20--60) each year, but remains a substantial cause of morbidity among children, resulting in approximately 55,000--70,000 hospitalizations, 205,000--272,000 emergency department (ED) visits, and 410,000 physician office visits (3). In the continental United States, rotavirus activity follows a distinct winter-spring seasonal pattern (4). In winter months, approximately 50% of hospitalizations and ED visits and 30% of outpatient visits for acute gastroenteritis among U.S. children aged <3 years are caused by rotavirus (5). To prevent rotavirus disease, in February 2006, a human-bovine rotavirus vaccine, RotaTeq® (Merck & Co., Inc., Whitehouse Station, New Jersey), was recommended for routine use among U.S. infants (3). To summarize rotavirus activity through May 3, during the current 2007--08 season, CDC analyzed data from the National Respiratory and Enteric Virus Surveillance System (NREVSS) and the New Vaccine Surveillance Network (NVSN). The results indicated that, when compared with the 15 previous seasons spanning 1991--2006, rotavirus activity during the current season appeared delayed in onset by 2--4 months and diminished in magnitude by >50%. Additional surveillance and epidemiologic studies are needed to confirm the impact of rotavirus vaccination on the 2007-08 season and to monitor the impact of the vaccine on the incidence and epidemiology of rotavirus during future seasons. NREVSS is a voluntary network of U.S. laboratories that provides CDC with weekly reports of the number of tests performed and positive results obtained for a variety of pathogens. For rotavirus, results of antigen testing using commercially available enzyme immunoassays (EIAs) are reported. Clinical and epidemiologic data are not obtained. During July 1991--June 2007, for each season, a median of 66 laboratories (range: 58--77) contributed rotavirus testing data to NREVSS. To approximate the median from previous seasons, 70 laboratories reporting directly to CDC were included in the 2007--08 analyses.* To compare detection rates of rotavirus during the 2007--08 season with prevaccine seasons, NREVSS data were aggregated by surveillance week for the period July 1991--June 2006 (i.e., maximum, median, and minimum) and compared with results for July 2007--May 3, 2008. Data from July 2006--June 2007 were excluded from the prevaccine (1991--2006) baseline data because some persons tested likely received vaccine during that period. To explore trends in rotavirus testing practices and results, additional comparisons were performed using only data from 32 laboratories that consistently reported >30 weeks of data per year during July 2000--June 2007 and reported >2 months during July 2007--May 2008. Since 2006, NVSN has consistently conducted prospective, population-based surveillance during January--May for rotavirus gastroenteritis among children aged <3 years residing in three U.S. counties (Monroe County, New York; Hamilton County, Ohio; and Davidson County, Tennessee). NVSN collects epidemiologic and clinical information on children with symptoms of acute gastroenteritis (i.e., diarrhea or vomiting) in inpatient, ED, and sentinel outpatient clinic settings. Fecal specimens are obtained and tested for rotavirus by commercial EIA tests (Premier Rotaclone, Meridian Biosciences, Cincinnati, Ohio). For this analysis, the number and proportion of acute gastroenteritis patients aged <3 years whose fecal specimens tested positive for rotavirus at NVSN sites during January--April in the years 2006, 2007, and 2008 were examined. Based on NREVSS data, the onset of national rotavirus activity during the 2007--08 season appeared delayed by approximately 2--4 months compared with the 15 prevaccine rotavirus seasons (July 1991--June 2006)† (Figure 1). During 1991--2006, median onset occurred in mid-November (week 46; range: week 41 to 52). In 2008, onset of rotavirus activity occurred in late February (week 9). The proportion of all rotavirus tests that were positive from mid-November 2007 to mid-April 2008 (week 46 in 2007 to week 16 in 2008) was below the minimum level reported during 1991--2006. Whereas in all previous seasons the proportion of tests that were positive peaked by March (week 12) to a median of 41.0% (range: 30.6%--45.5%), in 2008 only 13.5 % of tests were positive in week 12, and only 17.8% were positive at the season peak at the end of April (week 17). Since reaching that peak, the percentage of rotavirus positive tests has continued to decline. For the week ending May 31, 2008 (week 22), the proportion of tests positive for rotavirus was 11.1%. The delayed season and atypically low percentage of rotavirus-positive tests has been observed in all four U.S. census regions (6). Data from the 32 NREVSS laboratories that reported >30 weeks of data per year during July 2000--June 2007 and reported >2 months during July 2007--May 2008 were analyzed. During July 2, 2000--May 3, 2008, the 32 laboratories reported a total of 121,100 rotavirus antigen detection tests with 26,478 positive results (21.9%) (Figure 2). Although some year-to-year variation occurred during this period in the total number of tests and the number that tested positive for rotavirus antigen, both numbers were substantially lower during the 2007--08 rotavirus season than during any of the prevaccine seasons. When the total number of rotavirus tests performed during January 1, 2008--May 3, 2008 (weeks 1--18) was compared with the total number performed during these same weeks in each of the seven preceding rotavirus seasons, the number of 2008 tests was lower by a median of 37.0% (season range: 27.0%--45.9%). The number of tests that were positive for rotavirus was lower by a median of 78.5% (season range: 70.9%--79.7%). Similar declines were observed in all regions. In NVSN, 405, 481, and 283 children aged <3 years were enrolled during January 1--April 30 in 2006, 2007, and 2008, respectively. Among enrolled children, the overall percentage of fecal specimens testing positive for rotavirus was 51% in 2006, 54% in 2007, and 6% in 2008. Smaller percentages of positive results were observed at all inpatient, ED, and outpatient clinic sites in 2008 compared with 2006 and 2007 (Table). Reported by: National Respiratory and Enteric Virus Surveillance System. MA Staat, MD, G Fairbrother, PhD, Dept of Pediatrics, Univ of Cincinnati College of Medicine, Cincinnati Children's Hospital Medical Center, Ohio; KM Edwards, MD, M Griffin, MD, Dept of Pediatrics, Medicine, and Preventive Medicine, Vanderbilt Univ Medical Center, Nashville, Tennessee; PG Szilagyi, MD, GA Weinberg, MD, CB Hall, MD, Dept of Pediatrics, Univ of Rochester School of Medicine and Dentistry, Rochester, New York, New Vaccine Surveillance Network. CA Panozzo, MPH, DC Payne, PhD, JE Tate, PhD, HA Clayton, MPH, AL Fowlkes, MPH, M Wang, MPH, AT Curns, MPH, J Gentsch, PhD, MM Cortese, MD, M Patel, MD, MA Widdowson, VetMB, U Parashar, MBBS, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC. Editorial Note:In the United States, rotavirus activity during the ongoing 2007--08 season appears both substantially delayed in onset and diminished in magnitude, compared with previous years. These changes in rotavirus activity coincide with increasing use of rotavirus vaccine among infants. Although nationally representative data on vaccine coverage are not currently available, information from population-based immunization information system sentinel sites indicates that mean coverage with 1 dose of rotavirus vaccine among infants aged 3 months was 49.1% (range for six sites: 40.1%--65.4%) in May 2007 and 56.0% (range for eight sites§: 12.4%--75.8%) in March 2008 (7). Mean coverage with 3 doses of rotavirus vaccine among children aged 13 months at the sentinel sites was 3.4% (range: 0--11.0%) in May 2007 and 33.7% (range: 1.1%--53.0%) in March 2008 (D. Bartlett, MPH, CDC, personal communication, 2008). Most children aged >2 years at the start of the 2007--08 rotavirus season would not have received rotavirus vaccine because they would have been too old (e.g., >13 weeks) to start the series when the vaccine was first licensed in February 2006. Because the changes in rotavirus activity appear more pronounced than might be attributed to direct protective effects of vaccination alone, the results of this analysis suggest that vaccination of a proportion of the population might offer indirect benefits to unvaccinated children (i.e., herd immunity) by reducing transmission of rotavirus in the community. The findings in this report are subject to at least five limitations. First, the 2007--08 rotavirus season is still ongoing, and further information is needed to evaluate rotavirus activity fully. Second, although most laboratories submit reports to NREVSS within 2 weeks of testing, delays in reporting might have some effect on these preliminary data. Third, testing for rotavirus is not part of routine clinical practice and is conducted at the discretion of the physician and based on institutional policies. Changes in testing practices might impact these findings; however, such changes would be unlikely to explain the large decline in positive test results in 2008, particularly given the consistency of this decline across participating laboratories. Fourth, because NREVSS is a purely laboratory-based surveillance system, patient-level information is not available and NREVSS might receive more than one result for a given patient. However, any contribution of this to the results likely would be small. Finally, the counties where NVSN conducts active surveillance might not be representative of the entire U.S. population; however, the findings from NREVSS support very similar interpretation. The ongoing 2007--08 rotavirus season appears substantially delayed in onset and diminished in magnitude compared with previous seasons. These changes coincide with increasing use of rotavirus vaccine. Continued surveillance and additional epidemiologic studies are needed to confirm the effects of rotavirus vaccination on the 2007--08 season and to monitor the effects of the vaccine on the incidence and epidemiology of rotavirus disease over time. References
* For the 2007--08 season, a data-sharing agreement between CDC and Surveillance Data, Inc. (SDI) (Plymouth Meeting, Pennsylvania) increased the number of laboratories contributing rotavirus data to 214. SDI data shared with NREVSS showed patterns similar to data from other NREVSS laboratories; however, for sampling consistency, only 70 laboratories reporting directly to CDC were included in the 2007--08 analyses. Data from all 214 laboratories are available at http://www.cdc.gov/surveillance/nrevss/rota-data.htm. Additional information is available via e-mail at nrevss@cdc.gov. † Rotavirus national season onset was defined as the first of 2 consecutive weeks during which the median percentage of specimens testing positive for rotavirus antigen from all combined laboratory data is >10%. § Not all six sites reporting in 2007 were among the eight sites reporting in 2008. Figure 1 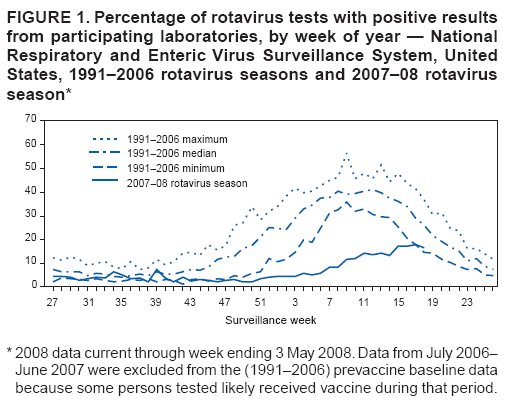 Return to top. Figure 2 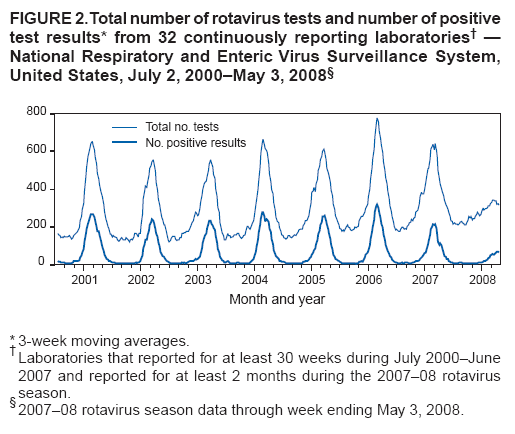 Return to top. Table 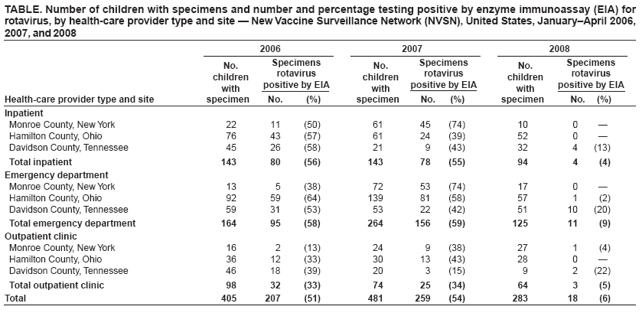 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/25/2008 |
|||||||||
|