 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Measles Elimination --- Japan, 1999--2008In 2005, the Regional Committee of the World Health Organization (WHO) Western Pacific Region (WPR) set a target date of 2012 for measles elimination in all WPR member states. In Japan, measles control strategies have included 1) a nationwide public awareness campaign implemented in 2001 to promote timely vaccination with the first dose of measles-containing vaccine (MCV1) administered on or after age 12 months, and 2) a 2-dose MCV schedule with the second dose (MCV2) administered at age 5--6 years, adopted in 2006 in accordance with the recommended WPR measles elimination strategy. However, during 2007--2008, Japan experienced a large measles outbreak, which resulted in exportation of measles cases from Japan into countries where measles elimination had been achieved. This report describes the epidemiology of measles in Japan during 1999--2008 and approval of a National Measles Elimination Plan in December 2007 that includes recommendations for immunization strategies, case-based measles surveillance, and monitoring to ensure elimination of measles by 2012. Measles continues to be endemic in Japan, with most cases occurring in children before school entry, except for 2007 and 2008, when a shift to an older age group was observed. With implementation of the National Measles Elimination Plan, Japan is expected to make progress toward achieving the WPR measles elimination goal. During 1999--2007, measles surveillance in Japan consisted of aggregate case reporting from two sentinel surveillance systems: pediatric and adult. In the pediatric sentinel system, cases were reported from a representative sample of approximately 3,000 pediatric inpatient and outpatient facilities. In the adult sentinel system, cases were reported from a sample of approximately 450 inpatient hospitals. In April 2006, the adult definition was changed from age >18 years to age >15 years; however, some pediatric sentinel sites continued to report cases in persons aged >15 years. For both pediatric and adult surveillance systems, the case definition for measles was the presence of a generalized rash, fever (101.3°F [38.5°C]), and cough, coryza, or conjunctivitis; or laboratory-confirmed measles. Laboratory confirmation of cases was performed by detection of measles-specific immunoglobulin M (IgM) antibodies, which was usually performed by commercial laboratories; virus isolation and genotyping were conducted by public health institutes in the country's prefectures (i.e., Japanese jurisdictions that are larger than districts and smaller than regions). During 2000--2007, the total number of pediatric measles cases was estimated using a multihypergenomic distribution by multiplying the average number of reported cases per sentinel medical facility by the total number of similar medical facilities nationally (1). For adult cases, estimates could not be calculated because sentinel hospitals were not chosen for representativeness. In January 2008, the two sentinel surveillance systems were replaced with nationwide case-based reporting of measles, and all health practitioners were required to report any clinical or laboratory-confirmed case to local health officials. Population immunity and vaccination coverage for eight vaccine-preventable diseases in Japan were measured by the National Epidemiological Surveillance of Vaccine-Preventable Diseases, an annual, national seroepidemiologic survey conducted among a representative sample of the Japanese population (2). Measles outbreaks occurred each year in Japan during 1999--2003 and involved both children and adults (Figure 1). The largest outbreak occurred in 2001, when the number of measles cases among children aged <15 years was estimated at 265,000. During 2002--2006, the number of reported pediatric measles cases decreased to a low of 516 in 2006. In 2001, national MCV1 coverage was estimated at 83.2% in children aged 24--35 months. In 2002, after a pediatrician-initiated nationwide public awareness campaign, estimated MCV1 coverage increased to 96.4% among children in the same age group and to 97.9% in 2007 (Table). In 2007, a measles epidemic occurred with an estimated 18,000 cases nationally among children aged <15 years. Initially, measles cases were reported primarily from Tokyo and Saitama prefectures, but then spread throughout Japan during a 10-day holiday (Golden Week) in May 2007, affecting all 47 prefectures. The epidemic continued in 2008 when, using the new nationwide case-based reporting system, a total of 9,631 measles cases were reported through June 22. Of these, 6,169 (64.1%) were clinical cases and 3,462 (35.9%) were laboratory confirmed. Cases were reported from all 47 prefectures but centered in the Tokyo metropolitan region, where 4,229 (43.9%) cases were reported, and in Hokkaido, with 1,344 (13.9%) cases (Figure 2). Persons aged >15 years accounted for 5,794 (60.2%) cases, with 2,584 (26.8%) occurring among youths aged 15--19 years. Among 6,919 patients with vaccination status reported, 2,540 (36.7%) had been vaccinated previously; of those, 2,436 (95.9%) had received MCV1, and 104 (4.1%) had received MCV2. In 2008, measles virus was identified in nasopharyngeal or blood specimens submitted from 141 patients with suspected measles. Genotype results were available from 104 (73.8%) specimens; 96 (92.3%) were genotype D5, five (4.8%) were genotype H1, and three (2.9%) were genotype A (all three specimens obtained from recently vaccinated patients) (3). Nine cases of measles encephalitis were reported in 2007, and five cases were reported during January--June 2008. Ages of persons with measles encephalitis ranged from 13 to 42 years (median: 23 years); no encephalitis deaths were reported. In response to the 2007 outbreak and to achieve the measles elimination goal agreed on by WPR member states, the Japanese government approved a 5-year National Measles Elimination Plan in December 2007. The plan includes a 3-part strategy: 1) intensified efforts to achieve high vaccination coverage among children and young adults, including a 5-year catch-up campaign that began in April 2008, targeting cohorts aged 13 years and 18 years with measles and rubella combined vaccine (MR); 2) establishment of a nationwide case-based measles-rubella surveillance system; and 3) establishment of a National Measles Elimination Council and local measles elimination councils to provide program monitoring and oversight. In addition to the 5-year catch-up MR campaign, education officials will review each child's vaccination status at school entry and during routine physical examinations during the school year, encouraging vaccination for those who are behind schedule and following up until children have received 2 doses of MCV. Although Japanese schools have no vaccination requirements for entry, a national advocacy and communication campaign also will be conducted to encourage timely administration of MCV1 at age 12--23 months and MCV2 at age 5--6 years, before entering primary school. Reported by: T Sunagawa, MD, PhD, T Shimada MD, K Ueno-Yamamoto, MD, K Yamashita, PhD, K Tanaka-Taya, MD, PhD, Y Tada, MD, PhD, Y Yasui, MD, PhD, T Matsui, MD, PhD, K Taniguchi, MD, PhD, J Kobayashi, MD, N Okabe, MD, PhD, Infectious Disease Surveillance Center, National Institute of Infectious Diseases, Japan. Unit of Expanded Programme on Immunization, World Health Organization Regional Office of the Western Pacific, Manila, Philippines. Vaccines and Biologicals Dept, World Health Organization, Geneva, Switzerland. Global Immunization Div, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC. Editorial Note:The resurgence of measles in Japan in 2007 had wide-ranging effects, both domestically and internationally. Japanese residents with measles exported the virus into countries where measles elimination had been achieved, including the United States and Canada (4,5). Anecdotal reports also indicate that some visitors to Japan from the United States and Taiwan were infected with measles virus and developed measles upon return to their home countries. The international spread of measles virus from Japan provides a reminder that countries in regions that have eliminated measles need to maintain very high levels of vaccination coverage and high-quality surveillance to limit the spread of imported measles virus. Virologic surveillance in Japan demonstrated a succession of genotypes since surveillance activities began there in the early 1990s. Genotypes D3 and D5 cocirculated for most of the 1990s, with genotype D5 more frequently detected in 2001 and genotype H1 during 2002--2005 (6,7). In 2006, genotype D5 apparently was reintroduced in Japan (8) and has been associated with measles cases imported from Japan into the United States (4,5). Effective implementation of the immunization strategies in Japan's National Measles Elimination Plan is aimed at reaching high vaccination coverage (>95%) among persons aged <22 years and also is expected to affect older age groups through herd immunity. The overall goal is to achieve elimination of measles by 2012. Monitoring disease incidence, surveillance quality, and vaccination coverage is critical to ensure progress toward elimination. Shifting to nationwide case-based surveillance was critical for Japan to progress toward measles elimination. Use of this system in 2008 enabled more representative reporting of adult cases, allowed for estimation of age-specific incidence, and provided information on the vaccination status of persons with reported measles. In addition to documenting the progress toward measles elimination, the nationwide surveillance system will monitor the impact of measles elimination activities on the incidence of rubella and congenital rubella syndrome. The WHO-recommended strategies for measles elimination include high routine coverage with 2 doses of MCV, supplementary immunization activities when routine coverage is not adequate, high-quality case-based measles surveillance, and access to a high-quality measles laboratory network. By adopting these recommended strategies, member states of WPR have made substantial progress in reducing the number of measles cases and deaths. Moreover, in 2006, South Korea became the first country in WPR to declare measles elimination (9). With Japan's renewed commitment and political and financial commitments from all WPR member states and partners, the region is progressing toward achieving the goal of measles elimination by 2012. References
Figure 1 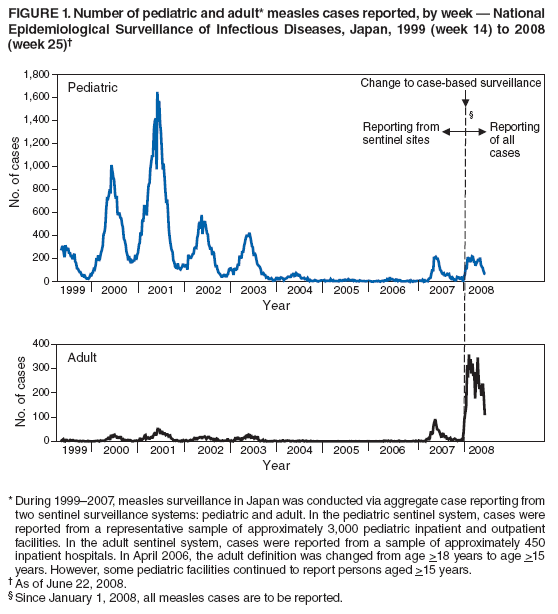 Return to top. Figure 2 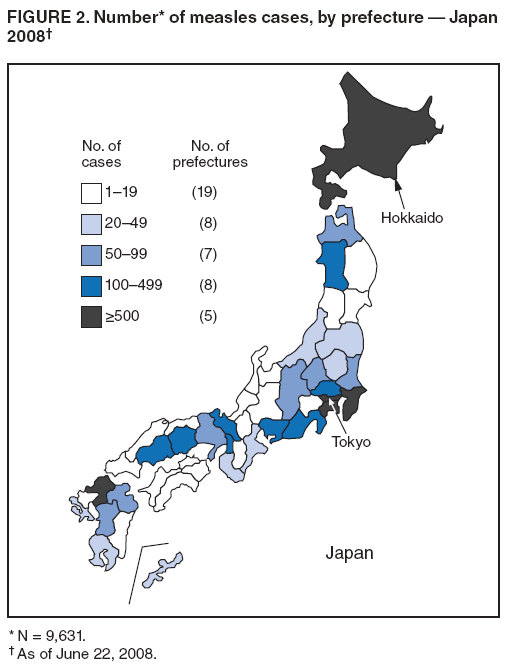 Return to top. Table 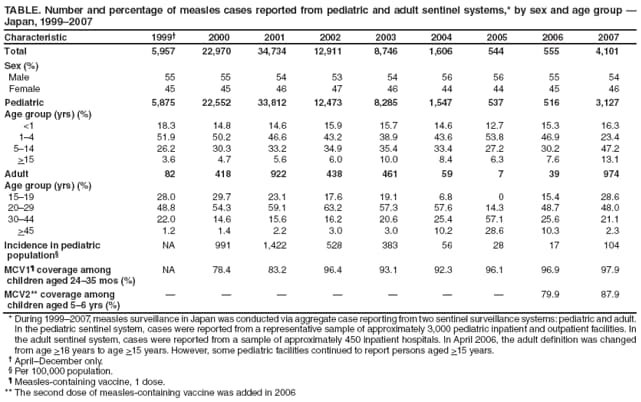 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 9/25/2008 |
|||||||||
|