 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
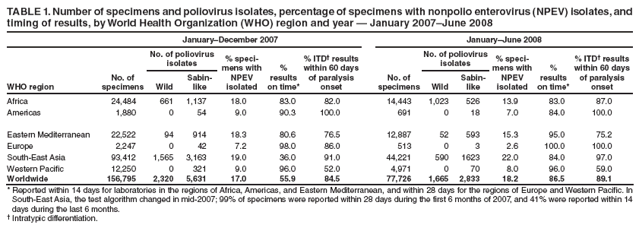
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Laboratory Surveillance for Wild and Vaccine-Derived Polioviruses --- Worldwide, January 2007--June 2008The Global Polio Laboratory Network (GPLN), comprising 145 facilities in 100 countries and operating in all six World Health Organization (WHO) regions,* was established in 1988 to support the Global Polio Eradication Initiative. GPLN isolates and characterizes polioviruses from stool specimens of patients with acute flaccid paralysis (AFP), from healthy contacts of AFP patients, and, in some laboratories, from sewage samples. Nucleotide sequences (viral capsid protein VP1 region; 900--906 nucleotides) are determined for wild poliovirus (WPV) isolates from each patient, contact, or sewage sample to target vaccination activities based on the patterns of virus transmission. This report updates previous reports (1,2) describing GPLN activities and vaccine-derived poliovirus (VDPV) surveillance during January 2007--June 2008. GPLN routinely screens for and characterizes VDPVs, which have caused polio outbreaks in areas with low oral poliovirus vaccine (OPV) coverage and caused prolonged infections in persons with primary immunodeficiencies (3). Data from GPLN guide the global initiative to eliminate polio. GPLN data are used to confirm polio cases, identify reservoirs of endemicity, determine serotype distributions of circulating polioviruses, detect importations, identify VDPVs, and ultimately document the absence of WPV and VDPVs for certification of polio eradication. Laboratory Network PerformanceWHO monitors GPLN performance through an annual laboratory accreditation program that uses proficiency testing, confirmatory testing by reference laboratories, and other measures to evaluate laboratory performance and the timeliness and accuracy of results. Of the 145 network laboratories, 143 were fully accredited in 2007, one was provisionally accredited, and one was not evaluated. Performance reviews are under way for 2008. GPLN tested 234,521 stool specimens from AFP cases during January 2007--June 2008 (Table 1), a 12% increase in workload compared with the previous 18-month period. Most (90%) AFP specimens were from the polio-endemic WHO regions of Africa, the Eastern Mediterranean, and South-East Asia, where workloads increased by 7.5%, 3.2%, and 23.8%, respectively. During mid-2006, GPLN began implementing measures to accelerate poliovirus confirmation in the 44 laboratories in polio-endemic regions. By June 2008, all 44 laboratories had adopted a new algorithm for virus isolation that shortened isolation reporting times from 28 days to 14 days. A new algorithm using polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay procedures for intratypic differentiation (ITD) between wild and vaccine-like polioviruses was introduced in 12 laboratories in mid-2006 and in an additional 10 laboratories by June 2008, and shortened ITD reporting times from 14 days to 7 days. During 2007--2008, the percentage of virus isolation results reported within 14 days of specimen receipt remained unchanged in the Africa region (83%), but increased from 36% to 95% in the Eastern Mediterranean region and from 36% to 84% in the South-East Asia region. Detection and Characterization of WPV Isolates and Transmission LinksWPV isolates were detected in stool specimens from AFP patients in 16 countries (4) during January 2007--June 2008 (Table 2). During this period, 1,257 (86%) of 1,470 WPV1 isolates and 2,444 (97%) of 2,518 WPV3 isolates were found in the four polio-endemic countries of Afghanistan, India, Nigeria, and Pakistan. In India, the ratio of WPV1 to WPV3 isolates reversed, from approximately 24:1 (July 2005--December 2006) to approximately 1:13 (January 2007--June 2008), reflecting a WPV3 outbreak, primarily in the states of Bihar and Uttar Pradesh, and also the effectiveness of program activities that prioritized the use of monovalent type 1 oral polio vaccine (mOPV1) in supplementary vaccination campaigns to interrupt WPV1 transmission. WPV1 endemicity is sustained in India by approximately six lineages, but the WPV3 outbreak has expanded the number of WPV3 lineages in India from approximately 10 to 40 (5). Sequence data reveal frequent cross-border transmission of WPV1 and WPV3 between southern Afghanistan and southern Pakistan, continued WPV1 endemicity in Sindh province in Pakistan, and persistent WPV1 and WPV3 circulation in and around Pakistan's Northwest Frontier Province, even as genetic diversity of WPV1 and WPV3 remains low (6). Although the number of cases in Nigeria were reduced by 50% during January 2007--June 2008 compared with July 2005--December 2006, multiple genetic lineages of WPV1 and WPV3 continue to circulate in the country (7). The 12 countries where polio is not endemic (Angola, Australia, Benin, Central African Republic, Chad, Democratic Republic of the Congo, Ethiopia, Myanmar, Nepal, Niger, Somalia, and Sudan) accounted for 212 (14%) of WPV1 isolates detected during January 2007--June 2008; all of these viruses were genetically linked to those found in India or Nigeria (Table 2). Sequence data showed that a WPV1 isolate of Indian origin detected in Angola in 2007 represented a continuation of an outbreak that began in 2005. WPV1 from the Angola outbreak spread to the Democratic Republic of Congo in 2007 and subsequently to the Central African Republic in 2008. A second WPV1 importation from India was detected in Angola in 2008. The WPV1 from Myanmar was imported from India via Bangladesh during 2006--2007, and the WPV1 from Somalia was imported from Ethiopia (with Nigeria as the ultimate source) during 2006--2007. Two genetic lineages of WPV1 circulated in Chad in 2007; one lineage signaled a new importation from Nigeria, with local spread, and the other signaled continued circulation of virus imported from Nigeria that had caused an outbreak in Chad more than 3 years earlier. WPV1 isolates detected in Ethiopia and Sudan in 2008 were of a different lineage than those detected in Chad, but were linked to each other and to outbreak viruses originating in Nigeria that were found at least 3 years earlier in these countries. WPV1 was isolated in Australia in 2007 from a Pakistani adult who had paralysis onset in Pakistan before traveling to Australia. The 12 countries where polio is not endemic also accounted for 74 (3%) of WPV3 isolates detected during January 2007--June 2008. WPV3 isolates in Angola and Nepal originated in India, and WPV3 isolates in Chad and Niger originated in Nigeria. In Nepal, seven importations of WPV3 were detected within 18 months, but no evidence of secondary spread was detected. However, local spread was detected from the WPV3 importations in Angola, Chad, and Niger. WPV1 and WPV3 also were detected by GPLN from non-AFP sources. WPV1 was isolated from a healthy contact of an AFP patient in Sudan in 2007. WPV1 and WPV3 found in sewage in Mumbai, India, in 2007 and 2008 were closely related to viruses circulating in the northeastern state of Bihar. WPV1 isolated from sewage in Switzerland in 2007 was closely related to WPV1 imported into Chad from Nigeria in previous years. No poliomyelitis cases were identified in Switzerland. Detection of VDPVsGPLN screens for VDPVs among vaccine-related isolates. Isolates are sequenced if results from ITD tests based on genetic and antigenic properties are discordant. A combination of sequence results, clinical status, and epidemiologic investigations are used to categorize Sabin-related isolates as 1) circulating VDPVs (cVDPVs), if obtained from two or more AFP cases in the same area; 2) immunodeficiency-associated VDPVs (iVDPVs), if isolated from persons with primary immunodeficiencies; or 3) ambiguous VDPVs (aVDPVs), if results provide no evidence of community circulation or immunodeficiency (3). During January 2007--June 2008, GPLN screened 8,478 Sabin-related isolates from AFP cases (Table 3). cVDPVs were found in Myanmar (eight type 1 from four cases) and Nigeria (207 type 2 from 101 cases).† iVDPVs were found in the Russian Federation (one type 1), Belarus (one type 2), and Iran (one coinfection with types 1 and 2, and one type 2). aVDPVs isolated in China in three areas (Guangxi Zhuang Autonomous Region [one type 1], Shandong Province [two type 1], and Shanxi Province [one type 1]) signaled independent events with no evidence of circulation. A type 2 VDPV isolated from a single AFP case in the Russian Federation in 2008 is under clinical investigation. A type 2 aVDPV was found in the Democratic Republic of Congo in 2007, and a type 3 aVDPV was detected in a child in Malawi in 2008. GPLN and collaborating laboratories also found type 1 aVDPVs in a sewage sample collected in Zurich, Switzerland in 2008, and type 2 aVDPVs in sewage samples collected in Egypt in 2007, in Israel in 2007 and 2008, and in Geneva, Switzerland, in 2008. No paralyzed persons have been determined to be associated with aVDPV detection in sewage. Reported by: Polio Eradication Dept, World Health Organization, Geneva, Switzerland. Div of Viral Diseases and Global Immunization Div, National Center for Immunization and Respiratory Diseases, CDC. Editorial Note:Results from GPLN regularly are used to target polio vaccination activities to interrupt poliovirus transmission. Data from GPLN also are evaluated to determine progress toward polio eradication, as indicated by reductions in geographic spread and reductions in genetic diversity among virus isolates. Based on these criteria, India made substantial progress in reducing WPV1 transmission during January 2007--June 2008. In contrast, WPV3 transmission was widespread in the Indian states of Bihar and Uttar Pradesh during most of this period, with genetic diversity increasing. Limited evidence of program progress was detected in Afghanistan, Pakistan, and Nigeria, the other three WPV-endemic countries (6,7). WPV importations continue because of failure to interrupt transmission in WPV-endemic countries, particularly Nigeria and India. A single infected traveler, such as the WPV1-infected person from Pakistan who went to Australia, is a source of virus who potentially can spread the virus within another country. This underscores the importance of maintaining laboratory and surveillance capacity in polio-free regions. Countries neighboring WPV-endemic countries (e.g., Chad, Niger, and Nepal) are at particular risk for repeated WPV importations, although long-range importations also occur, as was observed in the importation of WPV1 and WPV3 into Angola. The apparent lack of imported virus spread in Nepal demonstrates that high polio vaccination coverage can mitigate the potential consequences of importation. WHO estimated routine coverage with 3 doses of OPV by age 12 months in 2007 was 91% for Nepal, 36% for Chad, and 79% for Niger.§ Gaps in genetic information linking WPV isolates to their most closely related ancestor are interpreted as weaknesses in AFP surveillance. Substantial sequence gaps existed for viruses detected in Chad in 2007 and southern Sudan and Ethiopia in 2008. Such laboratory data are used to identify and address reasons for suboptimal surveillance performance. In Nigeria and Myanmar, locations overlapped where WPV and cVDPVs were found. In Nigeria, type 2 VDPVs cocirculated with WPV1 and WPV3, indicating serious problems with vaccination coverage. In Myanmar, local gaps in OPV coverage in 2007 allowed imported WPV1 to spread and type 1 cVDPV to emerge before both outbreaks were controlled. GPLN has reduced laboratory reporting time in polio-endemic regions by approximately 50% by implementing new test algorithms and increasing ITD testing capacity. The latter strategy required investments in staff training and equipment that will be offset by reductions in costly intercountry shipments of specimens. In some facilities, equipment provided for ITD testing also can be used for laboratory diagnosis of other vaccine-preventable diseases. * The six WHO regions are Africa, Americas, Eastern Mediterranean, Europe, South-East Asia, and Western Pacific. † The 207 cVDPV isolates were from 105 polio patients, four of whom had mixed WPV and cVDPV infections, and the paralytic disease was attributed to the WPV infections. § Available at http://www.who.int/vaccines/globalsummary/immunization/countryprofileselect.cfm. References
 Return to top. Table 2 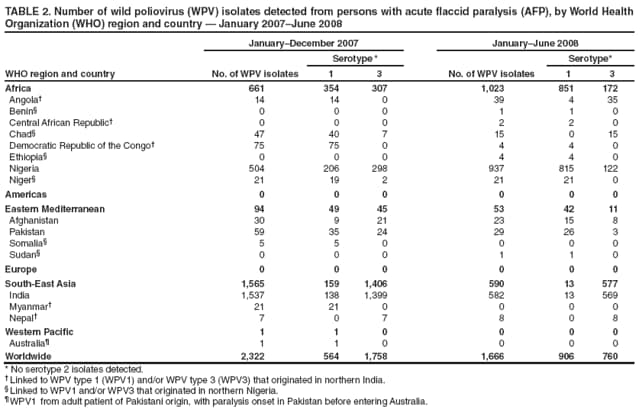 Return to top. Table 3 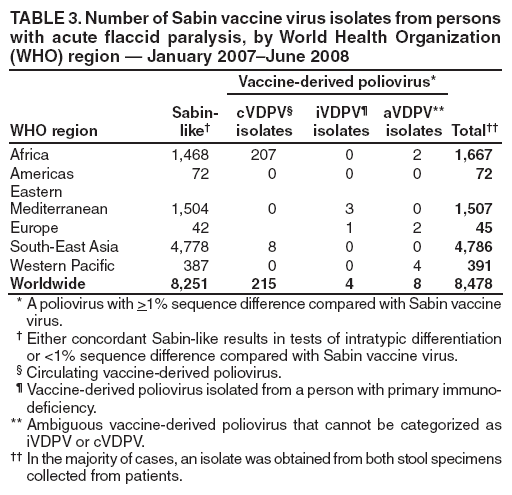 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 9/4/2008 |
|||||||||
|