 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Newborn Hepatitis B Vaccination Coverage Among Children Born January 2003--June 2005 --- United StatesHepatitis B vaccine was first recommended for administration to all infants in 1991 by the Advisory Committee on Immunization Practices (ACIP) as the primary focus of a strategy to eliminate hepatitis B virus (HBV) transmission in the United States (1). The recommended timing of administration of the first dose of hepatitis B vaccine to infants has evolved since then to optimize prevention of perinatal and early childhood HBV infections. In 1991, the first dose was recommended to be administered at birth before hospital discharge or at age 1--2 months. In 2002, ACIP indicated a preference for the first dose to be administered to newborns before hospital discharge (2). In December 2005, ACIP issued revised recommendations specifying that all medically stable newborns who weigh >2,000 g (4.4 lbs) receive their first dose of hepatitis B vaccine before hospital discharge (3). To measure hepatitis B vaccination coverage during the neonatal period, CDC analyzed data from the 2006 National Immunization Survey (NIS). This report summarizes the results of this analysis and provides national, state, and local data on vaccination coverage for infants who received the hepatitis B vaccine during the first days of life. The findings reveal that, during January 2003--June 2005, before implementation of the 2005 ACIP hepatitis B vaccine recommendation, the national newborn hepatitis B vaccination coverage estimate was 42.8% at age 1 day and 50.1% at age 3 days, with substantial variation by states and local areas. To comply with ACIP recommendations and increase coverage, delivery hospitals should provide hepatitis B vaccination of newborns as a standard of care. NIS provides estimates of vaccination coverage among noninstitutionalized children aged 19--35 months for each of the 50 states and selected local areas. To collect vaccination data, NIS conducts a random-digit--dialed telephone survey of households and a mail survey of children's vaccination providers identified by household respondents. Data are weighted to adjust for households with multiple telephone lines, household nonresponse, and exclusion of households without landline telephones (4). Infant age at vaccination was calculated by subtracting birth date from vaccination date. Children included in the 2006 NIS were born during January 2003--June 2005. Household response rate for the survey was 64.5%, based on Council of American Survey and Research Organizations guidelines (CASRO); 21,044 children with provider-verified vaccination records were included in this report and represent 70.4% of all children with completed household interviews. National newborn hepatitis B vaccination coverage was 42.8% at age 1 day, 48.5% at 2 days, 50.1% at 3 days, 51.1% at 4 days, 51.8% at 5 days, and 52.5% at 6 days. State and local area rates showed substantial variability, with hepatitis B vaccination coverage at age 1 day ranging from 8.2% in Fresno County, California, to 77.5% in Detroit, Michigan (Table). Among all states and local areas surveyed, the median coverage estimate was 50.3% at age 1 day and 58.7% at 3 days. Reported by: NJ Allred, PhD, N Darling, MPH, L Jacques-Carroll, MSW, EE Mast, MD, National Center for Immunization and Respiratory Diseases; SA Wang, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. Editorial Note:The analysis in this report indicates that, for the January 2003--June 2005 birth cohort, 42.8% of newborns had received hepatitis B vaccine by age 1 day and 50.1% had received hepatitis B vaccine by age 3 days. These data provide a baseline for assessing implementation of the December 2005 ACIP recommendation to administer hepatitis B vaccine to all newborns before hospital discharge (3). The 2009 NIS will be the first to include all survey-eligible children who were born after the December 2005 recommendation was made. Therefore, that survey will be the first to provide full estimates of national newborn vaccination coverage to evaluate the effect of the 2005 ACIP recommendation. Newborn hepatitis B vaccination coverage estimates varied substantially among and within states. Administration of hepatitis B vaccine to newborns is dependent on hospital policies and procedures and on provider and parent preferences (5,6). Although NIS does not distinguish whether hepatitis B vaccine was given before or after hospital discharge, National Hospital Discharge Survey data (7) indicate that the average length of hospital stay for all newborns in 2004 was 3.3 days, with an average stay of 2.1 days for well newborns and an average stay of 5.0 days for ill newborns; 85.6% of all newborns were discharged by age 3 days. The findings in this report are subject to at least four limitations. First, NIS is a telephone survey; although results are statistically adjusted to account for nonresponse and households without telephones, some bias might remain. Second, vaccination coverage is confirmed using provider-verified records. Although clinic providers might not always have records of a hospital-administered hepatitis B vaccine dose, this does not appear to result in substantial under-ascertainment of vaccination. A 2004 study in eight locations matched provider-reported vaccination records for the children sampled in NIS to their vaccination histories reported by the state Immunization Information Systems (IIS). NIS data underestimated birth dose coverage by no more than 5% at any one location when compared with the combined NIS and IIS coverage among children who had vaccination histories from both sources (M Khare, CDC, personal communication, February 2008). Third, estimates from state and local areas should be interpreted with caution because of smaller sample size and wider confidence intervals compared with the national estimate. Finally, infants who were not recommended to receive hepatitis B vaccine until age 1 month or after hospital discharge because their birth weights were <2,000 g and they were born to HBsAg-negative mothers could not be excluded from the coverage estimates. Inclusion of those infants in the denominator might result in an underestimate of newborn coverage, but the effect should be minimal because infants at this birth weight account for only 3% of births (8). Infants infected with HBV typically are asymptomatic and have a 90% likelihood of remaining chronically infected (3). Up to 25% of chronically infected children die prematurely of cirrhosis or liver cancer (9). Two primary modes of HBV transmission occur during infancy and early childhood: 1) from an infected mother to her infant during delivery, and 2) from infected household contacts to infant or child. Both modes of transmission can be prevented by immunization of newborn infants. For infants born to mothers identified as hepatitis B surface antigen (HBsAg)-positive (i.e., HBV-infected), administration of hepatitis B vaccine and hepatitis B immune globulin within 12 hours of birth is 85%--95% effective as postexposure prophylaxis in preventing HBV infection in the infant. In addition, hepatitis B vaccine alone is 70%--95% effective in preventing perinatal HBV transmission when the first dose is given within 24 hours of birth. Thus, administration of hepatitis B vaccine soon after birth provides timely postexposure prophylaxis to infants born to HBsAg-positive mothers who were not screened prenatally, or were not identified as HBsAg-positive because of testing errors or lapses in reporting or documentation of test results (10). Hepatitis B vaccination of all newborns also provides early preexposure protection to infants born to uninfected women during a period when the risk for developing chronic HBV infection is greatest. The 2005 ACIP recommendation to administer the first dose of hepatitis B vaccine to all newborns before hospital discharge will increase hepatitis B vaccination coverage during the first days of life. Delivery hospitals play a key role in the national strategy to eliminate HBV transmission. The 2005 ACIP statement recommends that delivery hospitals have policies and procedures in place, including appropriate standing orders, to ensure 1) administration of hepatitis B vaccine to all newborns with birth weights >2,000 g before hospital discharge and 2) identification of all infants born to HBsAg-positive mothers and infants born to mothers with unknown HBsAg status to allow initiation of postexposure prophylaxis within 12 hours of birth. State and local information on prevention of HBV infection in infants and children, including information on hospital-based policies and procedures to prevent HBV infection, is available through CDC-funded perinatal hepatitis B prevention coordinators based in state health departments. Contact information for those coordinators is available at http://www.cdc.gov/vaccines/vpd-vac/hepb/perinatal-contacts.htm. References
Table 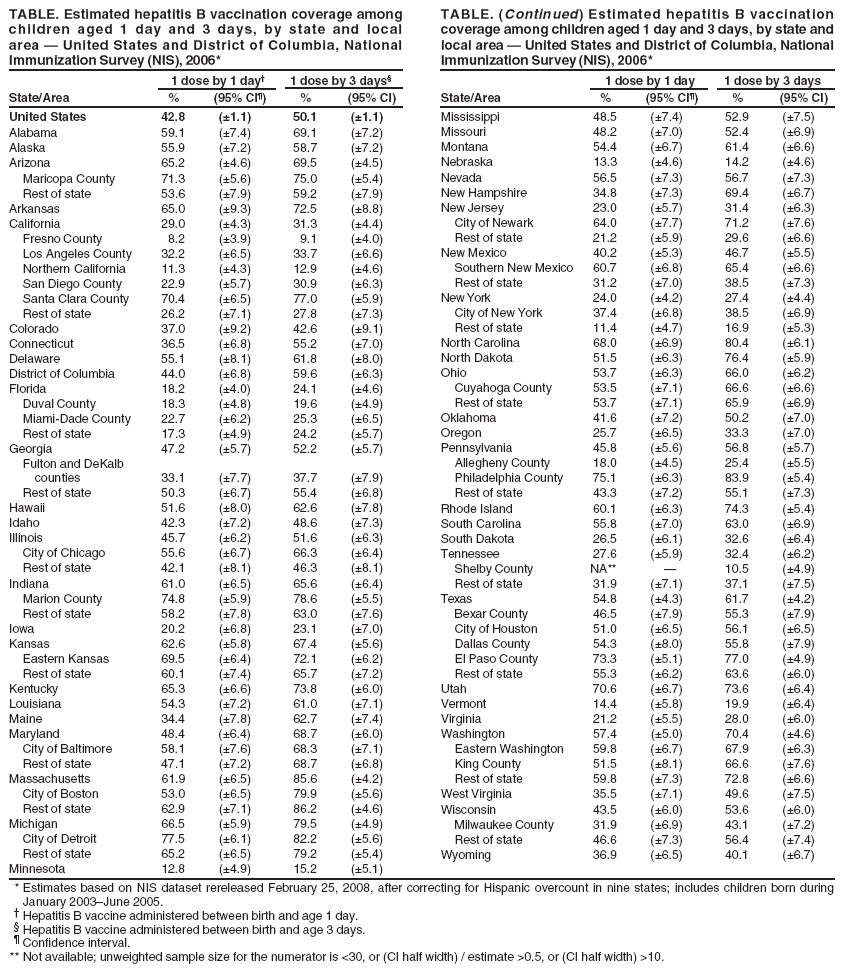 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 7/30/2008 |
|||||||||
|