 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Reporting of Chlamydial Infection --- Massachusetts, January--June 2003Chlamydia trachomatis infection is the most commonly reported sexually transmitted disease (STD) in the United States. An estimated 2.8 million infections occur annually (1). In 2002, a total of 834,555 cases in the United States, including 10,914 cases in Massachusetts, were reported through the National Notifiable Disease Surveillance System (NNDSS) (2). Chlamydial infection is most often reported in females, particularly those aged 15--24 years, reflecting a higher level of screening in females (3) but also important risk factors*. Although the majority of infections are asymptomatic, complications are potentially severe in women and include pelvic inflammatory disease, which can lead to tubal pregnancy, infertility, and chronic pelvic pain (3). Chlamydial infection during pregnancy can cause illness in the infant (e.g., conjunctivitis and pneumonia). Infection in men can manifest as urethritis and epididymitis. Timely, documented diagnosis and treatment of chlamydial infection are critical to prevent both complications and transmission. Since 1996, a progressive increase has occurred in the number of reported cases of chlamydial infection in Massachusetts (Figure 1), in part because of an increase in screening and use of more sensitive tests. This report summarizes an evaluation of chlamydial-infection reporting in Massachusetts during January--June 2003. The results underscore the need for improvement in both completeness and timeliness of reporting chlamydial infection in Massachusetts. Massachusetts law requires all laboratories (both in-state and out-of-state) and health-care providers (HCPs) to report within 24 hours evidence of chlamydial infection in any state resident (including college students from out of state). These data are used to assess contact follow-up and identify outbreaks. All cases are defined by laboratory diagnosis (e.g., isolation of C. trachomatis by culture, or detection of antigen or nucleic acid). Reports from both laboratories and HCPs are entered into STD*MIS, a database developed by CDC for use by state health departments. During January--June 2003, four laboratories in the state submitted their reports electronically to the Massachusetts Department of Public Health (MDPH). Certain laboratories reported daily by fax, mail, or electronically; others submitted their reports weekly or monthly, despite the requirement for 24-hour reporting. Laboratory reports do not contain information about indication for the test or treatment received. HCPs report to MDPH by using case-report cards, which include demographic, diagnosis, and treatment data. A morbidity record is created in STD*MIS by using data from whichever report (i.e., laboratory or HCP) is received first; the record is supplemented with data from whichever report is received second. Elimination of duplicate records is addressed through a combination of 1) systematic assessment of all laboratory reports and all HCP case reports for selected demographic indicators (i.e., patient name, address, and date of birth) as data are entered and 2) periodic (i.e., every 1--3 months) review of the database for duplicate records. Multiple records for the same person are considered to represent the same case of illness if the recorded dates of onset or diagnosis are within 14 days of each other (and considered as separate cases if those dates are >14 days apart). A disease-intervention specialist is assigned to follow up on a case-report card that indicates no evidence of treatment (even if no matching laboratory report exists). Because of resource limitations, positive laboratory reports without matching HCP case-report cards receive no follow-up from MDPH. Data reported during January--June 2003 from 48 laboratories and 510 HCPs in Massachusetts were evaluated for completeness and timeliness. Completeness of reporting was calculated by using the Sekar-Deming capture-recapture method (5), which compares data from two sources and estimates the number of cases missed by both sources and the estimated total number of cases. Timeliness of reporting chlamydial infection was determined by calculating the median number of days from laboratory specimen collection (for cases reported by HCPs) or date of positive laboratory test (for cases reported by laboratories) to date of reporting to MDPH for all reports submitted during January--June 2003. Capture-recapture calculations indicated that an estimated 1,757 cases of chlamydial infection were not reported to MDPH by either laboratories or HCPs during January--June 2003 and that the estimated total number of cases that occurred during that period was 7,016 (Figure 2). Using these values, the completeness of reporting was estimated at 46% for laboratory reports alone and 54% for HCP reports alone; 25% of cases were reported by both laboratories and HCPs. Using either laboratory and/or HCP reporting, the system accounted for 75% of the estimated total number of cases. In addition, of the 5,259 discrete cases reported to MDPH, 1,493 (28%) were reported only by laboratories and, therefore, did not receive case follow-up. Calculations of timeliness determined that median reporting times were 8 days for laboratories (interquartile range [IQR]: 6--13 days) and 12 days for HCPs (IQR: 8--20 days). Timeliness of electronic reporting versus paper-based reporting was not assessed in this study. Reported by: B Matyas, MD, T Bertrand, MPH, Y Tang, MD, B Dumas, S Ratelle, MD, A DeMaria, MD, Massachusetts Dept of Public Health. R Dicker, MD, Career Development Div, Office of Workforce and Career Development; D Katz, MD, EIS Officer, CDC . Editorial Note:The findings in this report identify the need to improve reporting of chlamydial infections in Massachusetts. Laboratories and HCPs are not reporting all of their cases, 28% of reported cases do not receive follow-up, and reporting is likely not timely enough to allow for intervention and to prevent transmission. Delayed reporting has a potential negative impact on the abilities of health departments to identify changes in disease trends and to conduct contact follow-up. Studies have demonstrated poor completeness of STD reporting and even worse timeliness among paper-based reporting systems (6). Electronic reporting by laboratories is more timely and complete than paper-based reporting and results in greater numbers of laboratory-based reports (7). Although laboratory reporting is considered more timely and complete, HCP reporting is still required by many states. A recent informal survey conducted by MDPH of the National Coalition of STD Directors of 16 project areas revealed that all areas required HCPs to report cases, most systems were paper-based, and response time required for both laboratories and HCPs differed by area (i.e., ranging from immediate to 7 days; one area had no time requirement) (MDPH, unpublished data, 2005). Although most project areas had not conducted formal evaluations of their surveillance systems, one of the 16 areas presently uses electronic reporting, and three of the 16 responding project areas are converting to Internet-based reporting. HCP reporting is still regarded as important because clinical data are generally absent from laboratory reports. As the electronic medical record becomes more common, linking laboratory reports to HCP electronic health records might become more feasible. The findings in this report are subject to at least one limitation. The capture-recapture method requires that the two systems being evaluated are independent, the study population is stable, and the events captured (e.g., diagnoses of chlamydial infection) are confirmed positives (8). Although HCPs are required to report presumptive cases of chlamydial infection, HCP reports were typically generated only after laboratory confirmation. This interaction might potentially increase the number of cases reported by both sources and the sensitivity of reporting from both sources. Therefore, the values obtained are likely overestimated. Multiple elements of the Massachusetts reporting system could be improved. For example, certain laboratories were unaware of the requirement to report chlamydial infections. As laboratories are identified through review of submitted HCP case reports, each laboratory might be contacted to ensure awareness of the requirement and that reports are submitted promptly. In addition, periodic surveys of HCPs might be conducted to identify which laboratories they use for diagnosis of chlamydial infection, with subsequent contact of those laboratories to ensure they are reporting. Public health surveillance systems should be evaluated periodically to ensure that problems of public health importance are being monitored efficiently and effectively (9). This recommendation from CDC (10) has been integrated into the core components and strategies being developed by the STD Division at MDPH. The requirement for laboratories to report a positive result within 24 hours will be maintained. As of June 7, 2005, a total of 10 laboratories in Massachusetts reported electronically, which should improve completeness and timeliness, key elements of the STD surveillance system that MDPH will continue to periodically evaluate. References
* Important risk factors include young age, being female (4), having new or multiple sex partners, inconsistent use of barrier contraceptives, cervical ectopy, douching, and low socioeconomic status.
Figure 1 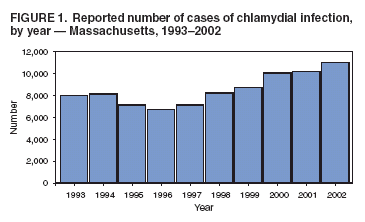 Return to top. Figure 2 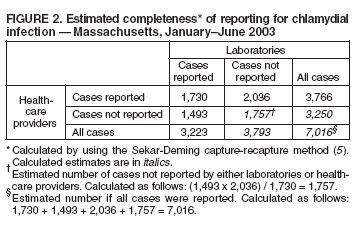 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/9/2005 |
|||||||||
|