 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Inadvertent Use of Bicillin® C-R to Treat Syphilis Infection --- Los Angeles, California, 1999--2004In March 2004, the Los Angeles County Department of Health Services (LACDHS) was notified that a large nonprofit clinic serving the gay and lesbian community in Los Angeles used a nonrecommended preparation of penicillin to treat syphilis patients during January 1999--March 2004. The clinic had inadvertently used Bicillin® C-R, a mixture of 1.2 million units (MU) benzathine penicillin G (BPG) and 1.2 MU procaine penicillin G, rather than Bicillin® L-A, a preparation that contains the 2.4 MU BPG per dose recommended by CDC (1). Bicillin L-A is recommended for treating syphilis and upper respiratory tract infections caused by susceptible streptococci (2). Bicillin C-R is indicated for streptococcal infections of the skin and respiratory tract; however, its efficacy in treating syphilis is unknown. The inadvertent use of Bicillin C-R, which contains only half the recommended dose of BPG for syphilis, was discovered after a patient treated for syphilis read the product insert, which stated that the medication was not indicated for treatment of syphilis. Review of clinic pharmacy records revealed that it received a shipment of Bicillin C-R in lieu of an unfilled order for Bicillin L-A in late 1998 and that the pharmacy subsequently ordered Bicillin C-R until March 2004. The clinic used Bicillin C-R as its exclusive formulation of injectable penicillin during January 1999--March 2004. This report summarizes the investigation of the misuse of Bicillin C-R at the Los Angeles clinic, which represents the largest occurrence of inadvertent treatment with Bicillin C-R to date. The investigation led to discussions among CDC, the Food and Drug Administration (FDA), and King Pharmaceuticals, Inc. (Bristol, Tennessee), whose Monarch Pharmaceuticals subsidiary markets Bicillin products. As a result, King Pharmaceuticals agreed to institute packaging and labeling changes to Bicillin products to prevent inadvertent treatment of syphilis with Bicillin C-R. Five BPG-containing products are marketed by Monarch: Bicillin L-A, Bicillin® L-A Pediatric (0.6 MU BPG), Bicillin C-R, Bicillin® C-R Pediatric (a mixture of 0.3 MU BPG and 0.3 MU procaine penicillin G), and Bicillin® C-R 900/300 (a mixture of 0.9 MU BPG and 0.3 MU procaine penicillin G). Despite a change in package color in 2002 to distinguish Bicillin C-R from Bicillin L-A (3), the proprietary names and package appearances remained similar for the two formulations (Figure). The product insert sheet included a warning against the use of Bicillin C-R for treatment of syphilis. Investigators reviewed databases from the clinic and from the LACDHS Sexually Transmitted Disease (STD) Program to identify patients who were treated during January 1999--March 2004 for confirmed syphilis infection or because of contact with a person known or suspected to have syphilis. All available data on treatment were evaluated. During January 1999--March 2004, a total of 429 patients were treated with Bicillin C-R for confirmed syphilis infection at the clinic. An additional 234 patients were treated with Bicillin C-R at the clinic for reported sexual contact with someone who was known or suspected to be infected with syphilis (contacts). Of persons with confirmed syphilis, none were female, and 215 (50%) were known to be infected with human immunodeficiency virus (HIV). Five (2%) contacts were female, and 10 (4%) contacts were known to be infected with HIV. No female patients were pregnant during or after treatment with Bicillin C-R. Clinic staff attempted to reach syphilis patients and contacts treated with Bicillin C-R by letter, up to three telephone calls, and, if necessary, telephone calls to emergency contacts listed on medical records. In addition, the clinic and LACDHS issued press releases to inform potentially affected patients and local health-care providers. LACDHS public health investigators attempted to reach patients whom the clinic was unable to locate or contact. A standard protocol was developed to retest and retreat all patients and contacts who had been treated with Bicillin C-R for syphilis. All patients were offered retreatment regardless of retesting results. Patients with a confirmed syphilis diagnosis were evaluated by clinic medical staff, retested with quantitative rapid plasma reagin (RPR) tests, and advised to undergo lumbar puncture for cerebrospinal fluid analysis if they had either clinical manifestations suggestive of neurosyphilis or evidence of treatment failure (e.g., less than a fourfold decline in RPR titer since initial treatment). Contacts were tested with a specific treponemal test, and those with a reactive test were managed in the same way as those with a confirmed syphilis diagnosis. Patients were offered retreatment with a CDC-recommended regimen appropriate for their stage of infection. As of January 26, 2005, of the 429 patients with confirmed syphilis, 282 (66%) were successfully contacted; 255 (59%) were retreated, 19 (4%) refused retreatment, and eight (2%) are pending evaluation. Of those who were retreated, 19 (4%) underwent lumbar puncture for suspected treatment failure. One patient treated for syphilis with Bicillin C-R subsequently had neurosyphilis diagnosed. Of the 234 contacts, 116 (50%) were successfully contacted, 98 (42%) were retested, and 15 (6%) are pending evaluation. Of the 98 contacts who were retested, 22 (22%) had serologic evidence of previous syphilis infection, and 19 (19%) were retreated; three (3%) refused retreatment. Operations at the clinic were disrupted for approximately 6 months. The clinic reassigned professional and clerical staff to the evaluation and retreatment effort, and some clinic activities were postponed or canceled. In addition, LACDHS dedicated two public health investigators to this effort for nearly 4 months. Reported by: R Bolan, MD, P Amezola, MPH, Los Angeles Gay and Lesbian Center; P Kerndt, MD, Los Angeles County Dept of Health Svcs, California. J Soreth, MD, Food and Drug Admin. M Taylor, MD, J Heffelfinger, MD, H Weinstock, MD, Div of STD Prevention, National Center for HIV, STD, and TB Prevention; M Greenberg, MD, M Janowski, MD, EIS officers, CDC. Editorial Note:Inadvertent use of Bicillin C-R for treatment of syphilis was documented in several STD programs during 1993--1998 (4). However, its misuse in treating approximately 660 persons in a Los Angeles clinic during January 1999--March 2004 is the largest reported occurrence to date and posed considerable clinical and programmatic challenges. Compared with procaine penicillin G, use of BPG results in detectable serum concentrations that are prolonged (up to 30 days for BPG, compared with up to 7 days for procaine penicillin G). Prolonged serum concentration is considered essential for treating syphilis effectively because sustained spirocheticidal levels are required to treat the slowly reproducing agent of syphilis, Treponema pallidum. Treatment of syphilis with half the recommended dose of BPG might have increased the risk for syphilis treatment failure and neurosyphilis, particularly among those infected with HIV (5--7). However, treatment failure and neurosyphilis can occur even with recommended penicillin regimens in persons with and without HIV infection (8). Therefore, whether treatment failures that occurred among those treated with Bicillin C-R represent an excess over what would be expected had they been treated with Bicillin L-A cannot be determined without additional data. An investigation by CDC and state and local health departments is assessing whether treatment failure was more common in patients treated with Bicillin C-R than in a similar population treated with Bicillin L-A. Such inadvertent use entails discomfort and inconvenience to patients because it requires retesting and possible retreatment for syphilis. In addition, inadequate treatment of syphilis might have contributed to an increase in the local transmission of the disease. In May 2004, CDC contacted FDA about the inadvertent use of Bicillin C-R. FDA worked with CDC and King Pharmaceuticals to design and implement changes to the product labeling, including more easily visible carton-color changes to distinguish L-A and C-R formulations, and the warning, "not for the treatment of syphilis," printed directly on syringes and cartons of Bicillin C-R. In November 2004, King Pharmaceuticals distributed a letter to clinicians, professional societies, and STD programs throughout the United States, alerting them to the potential for confusing Bicillin C-R with Bicillin L-A, the appropriate use of each formulation, changes in product labels, and mechanisms for reporting inadvertent use of Bicillin C-R for treatment of syphilis. Education of clinic managers, pharmacists, and providers in the proper use of different penicillin preparations might help reduce the inappropriate use of Bicillin products. Providers, STD clinics, and pharmacies should review their product records and tracking systems for ordering and delivering penicillin treatments for syphilis. References
Figure 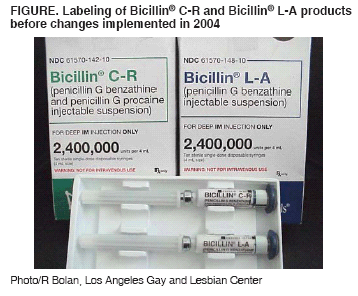 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/10/2005 |
|||||||||
This page last reviewed 3/10/2005
|