 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Outbreak of Invasive Pneumococcal Disease --- Alaska, 2003--2004In Alaska, statewide laboratory-based surveillance revealed an increase in invasive pneumococcal disease (IPD) in a rural region during 2003--2004. This report summarizes the outbreak, regional trends in serotype-specific pneumococcal carriage, and an assessment of use of standing orders for vaccination. The results of this analysis underscore the preventability of IPD and the importance of vaccination. Since 1986, the Arctic Investigations Program (AIP) at CDC has conducted laboratory-based surveillance for IPD in Alaska. Laboratories throughout Alaska are requested to send to AIP any isolate of Streptococccus pneumoniae recovered from a normally sterile site. AIP confirms the identity, determines the serotype and antimicrobial susceptibility of each isolate, and collects epidemiologic information for each case. OutbreakDuring January 2003--March 2004, a total of 14 cases of IPD (compared with a mean of 2.8 cases per year [range: zero to six cases] during 1986--2002) were reported in region A, a remote area of Alaska. The estimated population of region A is 10,000, and 80% of the residents in that region are Alaska Natives. Among the 14 patients, median age was 38 years (range: 1--72 years). Illnesses included cellulitis, septic arthritis, pneumonia with bacteremia, empyema, bacteremia, and meningitis; two patients died (Table 1). Serotype 12F (a serotype contained in the 23-valent pneumococcal polysaccharide vaccine [PPV-23]) was identified as the causative agent in nine (64%) of the 14 IPD cases. Pulsed-field gel electrophoresis (PFGE) patterns of the invasive 12F isolates were indistinguishable. All 12F isolates from region A had reduced susceptibility to trimethoprim/sulfamethoxazole but were susceptible to all other antibiotics tested. During 1986--2002, only two (2%) of 105 invasive 12F isolates collected throughout Alaska demonstrated reduced susceptibility to trimethoprim/sulfamethoxazole. The PFGE pattern of the 12F isolates in region A was unique, compared with a random sample of 23 12F isolates collected throughout Alaska since 1986. No cases of serotype 12F disease were reported in region A during 1996--2002; three cases of invasive serotype 12F disease were reported during 1986--1995. PFGE analysis revealed that these historic region A cases were not related to the 2003--2004 outbreak strain. Among the nine patients for whom PPV-23 was indicated, the indications included alcoholism (seven patients), generalized malignancy (one), and age >55 years (three). One of these nine patients had been vaccinated with PPV-23 during the preceding 5 years and contracted disease caused by serotype 7C, a serotype not included in the PPV-23 vaccine. Among the eight patients who had not been vaccinated during the preceding 5 years, seven (88%) had disease caused by serotypes contained in PPV-23: 12F (five), 7F (one), and 22F (one). Four (50%) of the eight had received influenza vaccination at least once during the 2 years preceding their pneumococcal infection, indicating that they had accessed the health-care system and an opportunity for pneumococcal vaccination potentially had been missed. The two deaths occurred in adults who had indications for, but had not received, pneumococcal vaccine. CarriageNasopharyngeal (NP) carriage of S. pneumoniae precedes the development of invasive disease. As part of an ongoing project to follow trends in pneumococcal carriage and antimicrobial resistance, AIP conducted voluntary communitywide NP swab surveys in four region A villages annually during 1998--2003 (1). NP carriage of S. pneumoniae in region A ranged from 32% to 43% of persons swabbed. NP carriage of serotype 12F increased substantially in 2003 (Table 2). PFGE patterns of colonizing 12F isolates collected during 2003 were identical to those of the outbreak strain. Pneumococcal VaccinationIn this outbreak, six (67%) of nine patients had indications for PPV-23 based on medical conditions independent of age. Because of this, region A health-care providers sought to improve PPV-23 vaccination rates among younger adults with risk factors through use of a vaccine standing orders program. In response to the region A outbreak, pharmacists and immunization coordinators from 16 inpatient and outpatient facilities (including two region A facilities) were surveyed in Alaska to evaluate use of vaccine standing orders programs. Eight sites reported using standing orders; all eight reported improved rates of vaccination after implementation of standing orders programs. Reported challenges with existing standing orders programs included increased nursing workload and difficulty accessing immunization records. Of the remaining eight facilities not using standing orders, 75% of those surveyed believed such programs would be beneficial but reported barriers to implementation (e.g., difficulty accessing immunization records and insufficient staffing). Reported by: G Jorgenson, PharmD, R Singleton, MD, Alaska Native Medical Center, Anchorage, Alaska. J Butler, MD, T Cottle, T Hennessy, MD, D Hurlburt, K Rudolph, PhD, D Parks, Arctic Investigations Program, National Center for Infectious Diseases; L Hammitt, MD, EIS Officer, CDC. Editorial Note:During 2003--2004, region A experienced an outbreak of IPD in which seven (50%) of 14 patients had indications for vaccination and had disease caused by a vaccine-preventable serotype. Pneumococcal carriage is a dynamic process, and carriage of specific serotypes in a population fluctuates over time. On a statewide level, during 1986--1990, 12F was the most common pneumococcal serotype isolated in Alaska Natives aged >2 years, accounting for 20.1% of IPD (2). However, during 1991--2000, the frequency of IPD caused by serotype 12F in this same population subset decreased to 2.2%. In the 2003--2004 region A outbreak, an increase in carriage of serotype 12F was temporally associated with an increase in serotype 12F IPD. The Advisory Committee on Immunization Practices (ACIP) recommends a one-time vaccination with PPV-23 for all persons aged >65 years on the basis of its effectiveness against pneumococcal bacteremia. One revaccination after >5 years is recommended for persons aged >65 years if the first vaccine was administered before age 65 years. Revaccination >5 years years after the first dose is also recommended for persons aged >2 years who are at high risk for invasive pneumococcal infection and who are likely to have rapid declines in pneumococcal antibody levels (3). Surveillance for IPD in Alaska has documented that Alaska Natives have one of the highest rates of IPD in the world (2,4). In addition, age-related increases in rates of IPD occur at a younger age among Alaska Native adults compared with non--Alaska Native adults. Because of these findings, the Alaska Division of Health and Human Services recommends that all Alaska residents receive PPV-23 beginning at age 55 years and be revaccinated every 6 years (5). In the region A outbreak, adequate vaccination might have averted 50% of IPD cases. A national health objective for 2010 is to achieve pneumococcal vaccination in 90% of adults aged >65 years (6). The national self-reported prevalence of pneumococcal vaccination among persons aged >65 years was 61.8% (95% confidence interval [CI] = 61.0--62.6) in 2002 (7). The corresponding rate for residents of Alaska was 59.8% (CI = 50.3--69.1) (7). On the basis of evidence that standing orders programs improve vaccination rates, ACIP strongly recommends standing orders for pneumococcal and influenza vaccinations in inpatient and outpatient settings, long-term--care facilities, managed-care organizations, assisted-living facilities, and home health-care agencies (7--9). Standing orders programs allow clinical staff to administer vaccinations according to an institution- or physician-approved protocol without the need for a physician's examination or direct order. Survey results suggest that successful standing orders programs depend on convenient access to reliable immunization records and adequate clinical staff support. When resources are available, computer-based standing orders effectively increase vaccination rates (10). In the case of missing immunization records, providers should follow the 1997 ACIP recommendations to vaccinate patients who are uncertain about their vaccination histories or have incomplete records (3). The 2003--2004 region A outbreak emphasizes the need to take every opportunity for vaccination in both inpatient and outpatient settings. Many patients with risk factors indicating vaccination might not have a regular primary-care provider but instead might seek medical attention in an emergency department or urgent-care clinic. Screening and subsequent immunization of persons with indications for vaccination in both primary-care and urgent-care settings could substantially reduce complications and death associated with pneumococcal disease. Region A initiated provider education and a standing orders program in response to the outbreak; surveillance for IPD continues. Other health-care providers, both in Alaska and nationally, should identify and address barriers to vaccination. Implementation of ACIP recommendations for standing orders programs is strongly recommended to take advantage of opportunities for vaccination and reduce pneumococcal morbidity and mortality. References
Table 1 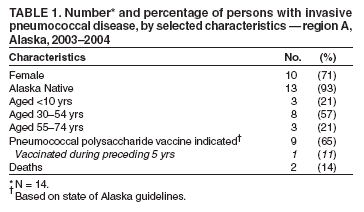 Return to top. Table 2 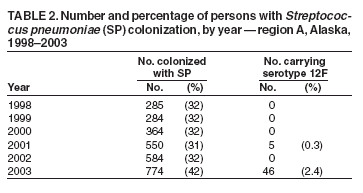 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/27/2005 |
|||||||||
This page last reviewed 1/27/2005
|