 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Acute Flaccid Paralysis Surveillance Systems for Expansion to Other Diseases, 2003--2004Since the 1988 World Health Assembly resolution to eradicate poliomyelitis, the number of countries where polio is endemic has decreased from 125 in 1988 to six at the end of 2003 (1). As part of the eradication strategy, a global surveillance system was established to 1) identify acute flaccid paralysis (AFP) cases in children aged <15 years and 2) deploy a network of accredited laboratories to perform virologic testing of stool specimens to determine whether the paralysis resulted from poliovirus infection. As AFP surveillance systems matured, countries increasingly applied AFP surveillance strategies and infrastructure to detect other diseases (2). This report describes the status of global AFP surveillance, including its expansion or use as a model in 131 (66%) of 198 countries for the reporting of measles and other vaccine-preventable diseases. As poliomyelitis is eradicated, AFP surveillance systems in these and other countries might be further expanded and adapted to improve the detection of and response to other diseases. AFP Surveillance SystemAny disease eradication initiative relies on sensitive and timely surveillance. Such surveillance is especially challenging for polio eradication, because only one of every 200 poliovirus infections results in clinically apparent paralytic disease. To ensure that paralytic polio cases will be detected if they occur, countries conduct surveillance for all AFP by using a standard case definition. All cases identified are tested to determine whether paralysis is caused by poliovirus infection. The quality of AFP surveillance is measured by using a standard definition for sensitivity and completeness, as follows: A rate of one or more nonpolio AFP cases per 100,000 population aged <15 years with timely collection of specimens indicates that surveillance is sensitive enough to detect polio and allows comparison of AFP reporting completeness among and within countries (Table). As of the end of 2003, a total of 196 of 214 countries and territories operated AFP surveillance systems* and reported data weekly to WHO. For many developed countries, the AFP surveillance system is integrated into existing disease surveillance systems. Countries with fewer public health resources receive external funding for polio eradication and to support a network of surveillance medical officers (SMOs). To promote quality AFP surveillance, SMOs maintain links to clinicians and the informal health sector (e.g., traditional healers, communities, and community informants). AFP field activities are supported by a three-tiered global polio laboratory network that operates in all six WHO regions and consists of 145 laboratories: 123 at the national level, 15 regional reference laboratories, and seven global specialized laboratories (Figure) (3). Network laboratories process stool samples from AFP cases to perform virus isolation, serotyping, intratypic differentiation, and genomic sequencing. A WHO-sponsored laboratory accreditation program monitors laboratory performance; in 2003, AFP surveillance in all six WHO regions met or exceeded performance standards, and 96% of network laboratories were fully accredited by WHO. During 2003, approximately 35,000 AFP cases were reported globally, with adequate stool specimens tested in 86% of all AFP cases (Table). A WHO region is certified polio-free after a period of 3 years without isolation of wild poliovirus from an AFP case, in the presence of high-quality AFP surveillance†. Three of the six WHO regions have been certified as polio-free. Expansion of Surveillance SystemMeasles. Globally, more than two thirds of countries with AFP surveillance have used that infrastructure, or applied it as a model, for measles surveillance (Table). As incidence of measles has declined to low levels, countries have shifted from aggregate measles reporting by age group to case-based reporting with laboratory confirmation. However, the extent of measles surveillance (i.e., as measured by the proportion of suspect cases that are tested) and the manner in which AFP strategies have been adapted for measles surveillance vary according to country resources and program goals (e.g., measles elimination versus mortality reduction) (Table). Consisting of 690 laboratories, the global measles laboratory network (Figure) developed much like the global polio laboratory network (4). The network's primary roles are serologic confirmation of suspected measles cases and genetic characterization of measles viruses. Measles laboratories have used much of the polio laboratory infrastructure; they are often housed at the same institutions and use similar systems for specimen transport, data management, communication, and reporting of results. Most network laboratories routinely test measles-negative sera for rubella and processed approximately 65,000 serum specimens from suspected measles cases in 2003 (Table). Measles laboratories also perform serologic diagnosis of yellow fever in countries in the Africa and Americas regions where yellow fever is endemic (Figure). In the Region of the Americas, the AFP surveillance system has been expanded or used as a model to fully implement case-based measles surveillance with laboratory confirmation of suspected cases. This approach has been instrumental in the successful interruption of endemic measles virus transmission (5). The system used in the Americas is now being expanded further to integrate rubella with measles surveillance in support of a regional goal to eliminate rubella and congenital rubella syndrome by 2010. In the Americas, in 2003, blood specimens were tested in 95% of suspected measles cases (Table). In other WHO regions, this proportion ranged from 1% to 29%. In polio-free countries in the African, Southeast Asian, and Eastern Mediterranean regions, polio-funded SMOs have conducted measles surveillance activities. In addition, measles funds support approximately 80 international and national staff members (e.g., epidemiologists, surveillance officers, data managers, and laboratory coordinators) and fund diagnostic kits and laboratory equipment. Measles program activities, including surveillance, have been supported by the Measles Initiative, which is supported by an international coalition§. Other Diseases. In 57 countries, neonatal tetanus (NT) is a major public health problem, causing 14% of all neonatal deaths (6); however, current reporting captures <5% of NT cases. With expansion of AFP surveillance programs, in certain countries, SMOs now search for cases of NT and other diseases in addition to cases of AFP when they visit health centers and hospitals. This active search identifies areas with NT cases and enables prioritizing areas for intervention through vaccination and education. In certain countries of the Africa Region, AFP surveillance provides a functional infrastructure, trained personnel, and other resources used to implement Integrated Disease Surveillance and Response (IDSR), a strategy adopted in 1998 by the Regional Committee of the WHO Regional Office for the Africa Region to strengthen all infectious disease surveillance activities, especially at the district level. This strategy includes integration of surveillance activities with laboratory support so that surveillance and laboratory data can be used to take specific and timely public health actions. As of June 2004, a total of 42 (91%) of 46 countries had completed surveillance assessments, 35 (76%) had adopted IDSR technical guidelines, and 44 (96%) countries had participated in a proficiency testing program through the National Public Health Laboratory in South Africa (7). Expansion of AFP surveillance systems has increased the responsibilities of SMOs in dozens of countries. In 2003, SMOs and polio/measles laboratory workers assisted in the detection and investigation of outbreaks of severe acute respiratory syndrome (SARS), cholera, dengue, Rift Valley fever, shigellosis, hemorrhagic fevers, meningitis, and malaria. SMOs conducted field and case investigations, collected samples, shipped them to laboratories, and organized outbreak response measures in coordination with local and regional health authorities. Laboratory workers processed the samples and reported the results locally and to regional networks as needed. Reported by: Immunization, Vaccines, and Biologicals Dept, WHO, Geneva, Switzerland. Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Global Immunization Div, National Immunization Program, CDC. Editorial Note:Adoption of the global polio eradication goal in 1988 required implementation of AFP surveillance in all countries and territories, including areas affected by conflict and other obstacles. As the AFP surveillance system matured, countries and regions began to use this system to conduct surveillance for other diseases. At present, two thirds of countries with AFP systems have adapted their systems for surveillance of measles and other vaccine-preventable diseases. The 2003 outbreak of SARS, the threat of influenza pandemics, and the importance of early detection of and response to outbreaks of other infectious diseases highlight the need for a more comprehensive global disease detection system. The urgent need for such a system is underscored by ongoing efforts to restructure the International Health Regulations (IHR) (8) as a framework for containment of global public health risks. In resource-poor countries and areas of conflict, the AFP surveillance system often is the only method for early detection of diseases that are prone to epidemics. External technical and funding support for AFP surveillance is provided by the international polio partnership¶. During 2003, of the more than $98 million provided for AFP surveillance by the partnership, $47 million was used for surveillance activity costs (e.g., operation of the laboratory network, transportation, communication, and meetings), and $51 million was used to pay approximately 2,700 international and national staff members who supported and conducted AFP surveillance and vaccination activities (Table). To date, diseases that have been successfully monitored by systems modeled after AFP and measles surveillance systems share common traits: well-defined case of syndromic presentation, relative ease of specimen collection for laboratory confirmation, strong international commitment and funding for control/elimination, and continued focus on using surveillance data for targeted control activities. The most obvious way to maintain and expand existing AFP and measles reporting systems is to phase in reporting of other diseases that support integration of surveillance activities. However, polio eradication must not be jeopardized by overburdening the systems. AFP and measles surveillance systems have the potential to serve as a foundation for a global network of public health laboratories that conducts surveillance for other infectious diseases. Expansion of these systems might encourage development of additional partnerships for global disease detection that will also help maintain the quality of future AFP and measles surveillance. References
* A total of 192 member states, one WHO associate, and 21 reporting entities report to WHO. Only 18 member states do not report AFP cases to WHO: Canada, Comoros, Denmark, Finland, France, Iceland, Japan, Luxembourg, Mauritius, Monaco, Netherlands, Reunion, San Marino, Saint Helena, Seychelles, Sweden, United Kingdom, and United States. † High-quality (i.e., certification-standard) AFP surveillance requires 1) detection of at least one case of nonpolio AFP per 100,000 population aged <15 years, 2) testing of two adequate stool specimens from at least 80% of AFP cases, and 3) testing of all specimens in WHO-accredited laboratories. § Consisting of WHO, UNICEF, the United Nations Foundation, the American Red Cross, the International Federation of Red Cross/Red Crescent Societies, the Canadian International Development Agency, and CDC. ¶ Led by Rotary International, WHO, UNICEF, and CDC. Table 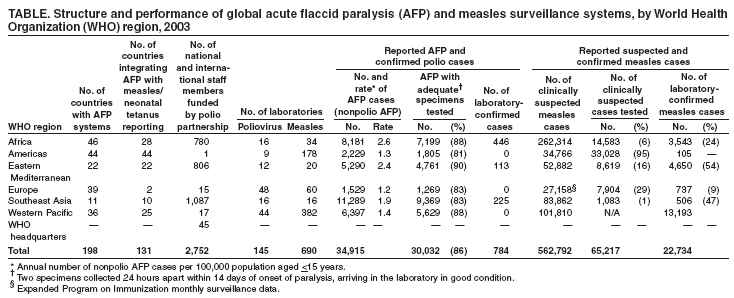 Return to top. Figure 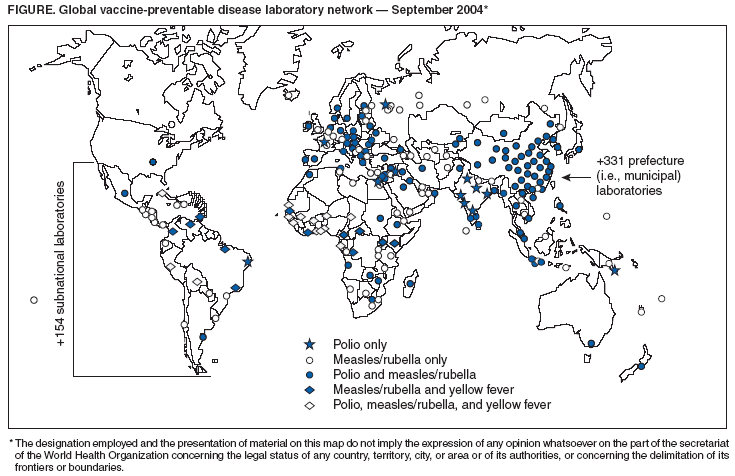 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/1/2004 |
|||||||||
This page last reviewed 12/1/2004
|