 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
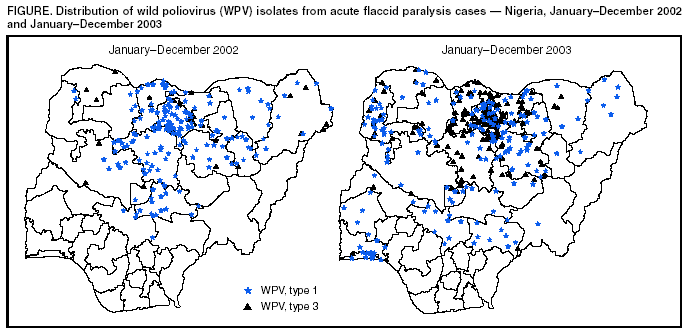
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication --- Nigeria, January 2003--March 2004Since the 1988 World Health Assembly resolution to eradicate poliomyelitis (1), three World Health Organization (WHO) regions (Americas, Western Pacific, and European) have been certified polio-free, and the number of countries where polio is endemic has decreased from 125 in 1988 to six in 2003 (Afghanistan, Egypt, India, Niger, Nigeria, and Pakistan). In 2003, Nigeria, the most populous country of the African continent (2003 population projected from 1991 census report: 125 million), reported 355 wild poliovirus (WPV) cases, accounting for 45% of cases reported globally and >80% of cases reported from the African Region (AFR). This report summarizes progress toward polio eradication in Nigeria during January 2003--March 2004. The findings indicate the urgent need to implement high-quality supplementary immunization activities (SIAs) in Nigeria to interrupt ongoing WPV transmission. Routine VaccinationTo date, routine coverage estimates vary considerably from state to state. Because administrative data for 2003 are still being evaluated, national estimates of coverage are not available. SIAsSince 1996, SIAs targeting children aged <5 years have been conducted annually in Nigeria (2). National Immunization Days (NIDs)* were conducted annually through 2002 (3). During February and March 2004, two NID rounds were conducted, targeting all 37 states (36 states plus one Federal Capital Territory [FCT]). All states except Kano and Zamfara participated in the February round, and all except Kano participated in the March round. In 2003, nine rounds of Subnational Immunization Days (SNIDs)† were conducted, targeting northern states where polio is endemic. The number of participating states and target population varied in each SNID, with the number of children vaccinated ranging from 3.6 to 15.0 million. Twelve states with endemic disease (Bauchi, Borno, Gombe, Jigawa, Kaduna, Kano, Katsina, Kebbi, Niger, Sokoto, Yobe, and Zamfara) and FCT participated in at least two rounds of SNIDs during 2003. Reported coverage at the state level during these SNIDs ranged from 56% to 100%. In addition, during 2003, four rounds of mop-up vaccination activities were conducted in Nasawara state and two rounds each in Benue and Kogi, sites that had been re-infected with WPV after being polio-free for >12 months. Reported coverage in these states during these mop-up activities ranged from 86% to 100%. National polio eradication programs analyze the oral polio vaccine (OPV) vaccination status (routine and supplemental doses) of children aged <5 years, with nonpolio acute flaccid paralysis (AFP) as a proxy for OPV coverage in the general population. As the OPV coverage of population increases, the percentage of nonpolio AFP cases with >3 doses of OPV also should increase. During 2003, the proportion of nonpolio AFP cases in children aged <5 years who had received >3 doses of OPV was <60% in 12 of 13 states (median: 33%; range: 9%--75%) where polio is endemic. The proportion of nonpolio AFP cases in children aged <5 years who had received >3 doses of OPV was <60% in five of eight re-infected states, but in only one of the 14 states without endemic disease. AFP SurveillanceSurveillance for AFP, initiated in 1997, is conducted at 4,035 reporting sites in the 774 Local Government Areas (LGAs) in Nigeria. AFP surveillance quality is evaluated by two key indicators: 1) annual reporting rate (target: nonpolio AFP rate of >1 case per 100,000 children aged <15 years) and 2) completeness of stool specimen collection (target: two adequate specimens from >80% of all persons with AFP). In 2003, Nigeria attained a national nonpolio AFP rate of 5.5; all 37 states attained rates of >1.0. The adequate stool specimen collection rate nationally for 2003 was 91%; all 37 states the target rate of >80% (Table). Surveillance performance at the LGA level varied; 123 (16%) of 774 LGAs were below the target levels for one or both surveillance indicators. When surveillance quality is high, previous genetic analyses of WPV from all regions with endemic disease have indicated that nearly all isolates are >99% identical to some other previous isolate in the viral VP1 coding region. During 2003, approximately 17.5% of the isolates in Nigeria (type 1 [PV1], n = 43; type 3 [PV3], n = 19) were <98.5% identical to any other previous isolate, indicating that some of the closely related polioviruses were not detected because of gaps in surveillance quality (i.e., viruses missed in the chain of transmission for >1 year). Stool samples collected from persons with AFP in Nigeria are tested at two WHO-accredited national polio laboratories, one in Ibadan (Oyo state) and one in Maiduguri (Borno state). In 2003, the Ibadan and Maiduguri laboratories processed 6,549 stool specimens. The proportion of specimens with nonpolio enterovirus (NPEV) isolated is used as a combined indicator of quality of specimen transport and sensitivity of laboratory processing; a rate of >10% is considered acceptable. The NPEV isolation rate during 2003 was 11.9% for Ibadan and 9.5% for Maiduguri. Median times for reporting primary isolation and intratypic differentiation results were 17 days (75% within 20 days) and 16 days (75% within 22 days), respectively. WPV IncidenceDuring 2002--2003, the number of confirmed WPV in Nigeria increased from 202 to 355 (Table). Of these, 192 were PV1, and 163 were PV3. In 2003, a total of 23 of 37 states reported at least one WPV, representing a wider area of circulation than in 2002, when 15 states reported WPV (Figure). Of these 23 states, 13 are considered to have endemic transmission, whereas 10 were re-infected after being polio-free for >12 months. Early in 2004, PV1 was reported from Anambra state, one of 14 southern states that had remained polio-free in 2003. In 2003, the outbreaks in Nigeria centered in Kano. Of 89 WPV cases in Kano, 57 (64%) were associated with PV3 and 32 (36%) with PV1. Virus sequence data indicated that the PV3 virus radiated outward along multiple independent chains of transmission. This outbreak started in March. The PV1 outbreak started in May, at the onset of high transmission season. A second peak of PV3 cases occurred in August, when numbers of PV1 and PV3 cases were equal. Of 355 polio cases reported in 2003, a total of 81 (23%) occurred in children aged >3 years, of which 69 (85%) were either never or incompletely vaccinated. Of the 18 genetic clusters (corresponding to groups of related chains of transmission) observed in Nigeria in 2002 (14 PV1 and four PV3), seven were not observed in 2003 (six PV1 and one PV3). However, the large outbreaks in 2003 have increased the genetic diversity of several clusters such that some previous PV1 clusters have expanded into at least four new genetic clusters, indicating intense transmission. Reported by: Federal Ministry of Health, Country Office of the World Health Organization, Abuja, Nigeria. Vaccine Preventable Diseases, World Health Organization Regional Office for Africa, Harare, Zimbabwe. Vaccines and Biologicals Dept, World Health Organization, Geneva, Switzerland. Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Global Immunization Div, National Immunization Program, CDC. Editorial Note:After gains toward polio eradication during 1996--2002, Nigeria suffered a resurgence of WPV transmission attributable to the suspension of vaccination campaigns in fall 2003 in several northern states, particularly Kano. This resurgence resulted in the reintroduction of WPV into previously polio-free Nigerian states and exportation of WPV to eight polio-free countries in West and Central Africa (Benin, Cameroon, the Central African Republic, Chad, Ghana, Ivory Coast, Burkina Faso, and Togo). The increased intensity of WPV transmission in the states with endemic polio in northern Nigeria in 2003 occurred despite an increased number of targeted SIAs. Seven of the 13 states with endemic disease were involved in four or more SNID rounds in 2003; all 13 states have continued to confirm WPV. Continued WPV transmission with expansion of genetic diversity, the occurrence of polio in older children, and OPV data from nonpolio AFP cases demonstrate the failure to reach a substantial proportion of children during the vaccination campaigns. False rumors about OPV safety adversely affected SNIDs, with the greatest impact in Kano, where 25% of all
Nigerian WPV cases occurred in 2003. Citing vaccine safety concerns, state authorities in Kano (which last conducted a SNID in
April
On January 15, 2004, the federal minister of health signed the Geneva Declaration on Polio Eradication together with the ministers from the other five countries with endemic disease. To address OPV safety concerns, the president of Nigeria established a safety verification committee, which included religious and traditional leaders, state officials, and federal government scientists. The committee undertook testing of OPV in India and South Africa and, in March 2004, presented its report, which indicated that OPV is safe. As a result, the northern states, except Kano, fully endorsed their participation in SIAs, with the president of Nigeria personally launching the campaign in Zamfara. Nigeria and its polio partner agencies have endorsed a strategic plan that proposes six SIA rounds in all states with endemic disease by December 2004. The plan focuses on improving the quality of microplanning, vaccination team selection, training, and social mobilization. In addition, a new mechanism for rapidly disbursing funds to vaccinators is being planned for 2004. Restoring public confidence in the safety of OPV will be critical to the success of SIAs. During 2003, Nigeria maintained certification-quality AFP surveillance at the national and state levels. Remaining gaps at the LGA level will be addressed through greater scrutiny of the timeliness and completeness of surveillance reports and prioritized supervision of low-performing LGAs. The opportunity to achieve polio eradication globally has never been greater. The government of Nigeria and its partners must ensure that every child is administered OPV, so that Nigeria, the rest of Africa, and the world can achieve polio eradication. References
* Nationwide mass campaigns during a short period (days to weeks) during which 2 doses of OPV are administered to all children (usually aged <5 years) regardless of previous vaccination history, with an interval of 4--6 weeks between doses. † Campaigns similar to NIDs but confined to certain parts of the country.
 Return to top. Table 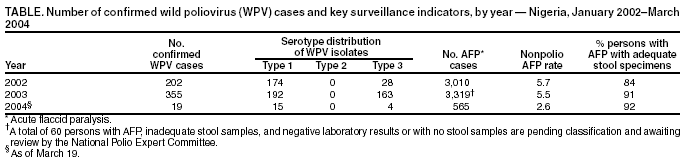 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/29/2004 |
|||||||||
This page last reviewed 4/29/2004
|