 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: West Nile Virus Screening of Blood Donations and Transfusion-Associated Transmission --- United States, 2003In 2002, transfusion-associated transmission (TAT) of West Nile virus (WNV) infection acquired through blood transfusion marked the emergence of a new threat to the U.S. blood supply (1). Although mosquito-borne transmission remains the predominant mode of WNV transmission (2), identification of TAT underscored the need for WNV screening of donated blood. In June 2003, blood-collection agencies (BCAs) implemented investigational WNV nucleic acid--amplification tests (NATs) to screen all blood donations and identify potentially infectious donations for quarantine and retrieval. This screening was performed on approximately 6 million units during June--December 2003, resulting in the removal of at least 818 viremic blood donations from the blood supply. This report summarizes the results of blood-donation screening tests conducted during 2003 and describes six cases of WNV TAT that occurred because of transfusion of components containing low levels of virus not detected by the testing algorithm. These data indicate that blood screening for WNV has improved blood safety. However, a small risk of WNV transfusion-associated transmission remains. To address this risk, changes to screening strategies are planned for 2004. BCA Testing ActivitiesIn June 2003, under the Food and Drug Administration's (FDA) investigational new drug (IND) mechanism, BCAs began screening donations by using NATs from two test-kit manufacturers. Initial screening protocols included NAT performed on mini-pools (MP NAT) of samples from six or 16 donations, depending on the test-kit manufacturer. Donation samples that were part of reactive mini-pools were tested individually. Any reactive samples were retested by individual donation testing (IDT NAT). In certain cases, an alternate sample from the same donation or an alternate NAT might have been used for retesting. In addition, selected blood banks serving areas with epidemic activity stopped using this MP NAT screening algorithm and implemented IDT NAT screening during limited periods of the epidemic season. Donors of IDT NAT--reactive samples identified by either screening method were asked to participate in a BCA-directed follow-up study to confirm WNV infection and evaluate for the persistence of WNV RNA in blood samples collected subsequently. Both follow-up samples and the index-donation samples were tested for WNV-specific IgM antibody. Donations that were IDT NAT--reactive were not released for transfusion; these donors were deferred from donating blood again until >28 days after the date of collection for the last NAT-reactive sample and the documented development of WNV-specific antibody. To determine the sensitivity of the MP NAT--screening algorithm, certain BCAs performed retrospective testing studies in selected areas that experienced high rates of viremic donations. In these studies, individual components of archived MP NAT--negative donation samples were retested by IDT NAT. Surveillance ActivitiesFor surveillance purposes, a donation that was repeatedly reactive on IDT NAT was considered to be from a presumptive viremic donor (PVD). Cooperating local blood centers provided reports of PVDs (including donor age, sex, postal code, and date of donation) to state health departments, which provided reports to ArboNET, the national arbovirus surveillance system. As of March 31, 2004, state and local health departments had reported 818 PVDs to ArboNET; dates of collection ranged from June 25 to December 2, 2003 (Figure). Complete information was available for 811 (99%) of these PVDs; six (1%) had West Nile viral encephalitis or meningitis subsequent to donation (median age: 45 years, range: 28--76 years), 137 (17%) had West Nile fever (median age: 46 years, range: 17--76 years), and 654 (81%) remained asymptomatic. Of the PVDs reported to ArboNET, 691 (85%) were residents of nine states (Colorado, Kansas, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming). These states experienced WNV epidemics in 2003 and accounted for 60% of reported cases of West Nile viral encephalitis or meningitis. WNV Transfusion-Associated Transmission InvestigationsSince 2002, public health authorities have been encouraged to investigate reports of WNV illness among patients who had received blood transfusions <4 weeks before illness onset and to report these suspected TAT cases to CDC. A probable TAT was defined as transfusion to a recipient who 1) had a confirmed WNV infection (3) and 2) had received a blood product from a NAT-reactive index donation associated with a donor with WNV-specific IgM antibody in the index donation or a follow-up collection. A confirmed TAT case was defined as meeting the criteria for a probable case and having any one of the following criteria: 1) unlikely mosquito exposure during the 14 days before recipient illness onset; 2) testing of remaining diagnostic samples from the hospitalized transfusion recipient indicating that WNV infection occurred at the time of transfusion; or 3) transfusion of a co-component of the infectious donation into another recipient who then had a confirmed WNV infection. A case was classified as a noncase if WNV infection could not be confirmed in the recipient <4 weeks after the implicated transfusions, if WNV RNA was not identified in any implicated donation, or if all implicated donors were seronegative for WNV. If samples were not available to satisfy the criteria for probable, confirmed, or noncase classification, the case was considered inconclusive. During 2003, a total of 23 suspected cases of WNV TAT were reported to CDC. Public health authorities reported 15 suspected cases of WNV TAT among patients who had WNV illness after receiving transfusions. Another eight suspected cases were in recipients of components derived from low-level viremic donations that were identified during special retrospective studies of MP-negative blood retested with IDT NAT by two BCAs. Follow-up of these eight cases was performed to determine if WNV infection had resulted from the implicated transfusions. As a result of these 23 investigations, six cases were classified as confirmed or probable WNV TAT, 11 as noncases, and three as inconclusive. As of March 27, 2004, three cases remained under investigation. In each of these six confirmed or probable cases, the recipient received components from multiple donations; however, only one infectious blood component was found in each case. All six of these infectious donations had been collected during July 29--September 18, 2003, and were not identified in MP screening. The median age of the six recipients was 63 years (range: 13--82 years); four had WNV encephalitis, one had West Nile fever, and one critically ill patient did not have discernible WNV-compatible illness despite confirmed WNV infection. A sufficient index-donation sample was available to estimate the titer of the implicated donor's viremia in four of six cases: the median estimated viremia was 0.11 plaque-forming units per milliliter (pfu/mL) (range: 0.06--0.5 pfu/mL). Two of these six cases were reported previously (4); a description of a third case follows. On August 31, 2003, a male aged 13 years was admitted to a hospital with multiple injuries. On September 1, he received three units of packed red blood cells. On September 9, after hospital discharge, he had a maculopapular rash. On September 12, he was readmitted to the hospital with fever, headache, vomiting, and diarrhea, consistent with West Nile fever; blood drawn on that day was positive for WNV-specific IgM antibody. The three transfused blood units had been collected during the second week of August 2003. No donors of this blood reported symptoms of WNV illness before or after donation. Samples from these donations were nonreactive for WNV RNA by MP NAT performed on six-specimen mini-pools. All other components derived from these three donations were quarantined immediately; there were no co-component recipients. Recalled plasma samples from the three index donations were WNV IgM negative. One donor seroconverted evidenced by development of WNV-specific IgM antibody in serum collected 50 days after donation. Recalled plasma from this donor was reactive when tested by IDT NAT. CDC confirmed results by using polymerase chain reaction; the estimated viral load was 0.09 pfu/mL. The recipient recovered without sequelae. Reported by: S Kleinman, MD, American Assoc of Blood Banks, Victoria, British Columbia, Canada. M Busch, MD, Blood Systems Research Institute, San Francisco, California. S Caglioti, Blood Systems Laboratories, Tempe, Arizona. SL Stramer, PhD, R Dodd, PhD, American Red Cross, Gaithersburg, Maryland. DM Strong, PhD, Puget Sound Blood Center, Seattle, Washington. W Dickey, MD, Belle Bonfils Memorial Blood Center, Denver, Colorado. B Salvidar, MS, M Gilchrist, PhD, Univ of Iowa Hygienic Laboratory, Iowa City; S Brend, MPH, Iowa Dept of Public Health. H Nakhasi, PhD, J Epstein, MD, J Goodman, MD, Center for Biologics Evaluation and Research, Food and Drug Administration. M Chamberland, MD, M Kuehnert, MD, Div of Viral and Rickettsial Diseases. L Petersen, MD, N Crall, A Marfin, MD, Div of Vector-Borne Infectious Diseases, National Center for Infectious Diseases; T Boo, MD, S Montgomery, DVM, EIS officers, CDC. Editorial Note:Previous studies have documented that an estimated 80% of WNV-infected persons remain asymptomatic but are believed to have viremia lasting a median of 6.5 days (5,6). Asymptomatic WNV-infected persons with viremia likely represent the largest risk group of blood donors. Because symptom screening at the time of blood donation will not identify most viremic donors, screening by NAT was implemented rapidly to identify potentially infectious blood donations by detecting WNV RNA. Use of blood-donor screening for WNV by NAT under the IND mechanism has enhanced the safety of the blood supply. Despite this enhanced safety, documentation of the six WNV TAT cases in 2003 indicates that blood components containing low levels of virus might escape detection and that at least some of these might be infectious. Virus loads in infectious donations were considerably lower in 2003 than in 2002 (1). In 2002, the estimated viremia levels in implicated donations were 0.8--75 pfu/mL, compared with 0.06--0.5 pfu/mL for TAT cases during 2003. The reasons for this lower range are unclear, and the lower limit of donor viremia that can lead to transfusion-associated infection is unknown. Data collected during 2003 will be considered by the blood supply community in collaboration with public health authorities when developing screening strategies for 2004, when widespread seasonal transmission of WNV is expected to continue. MP screening will continue to identify most persons who donate during the short viremic period, but prospective IDT might be implemented in regions with high WNV-infection rates (i.e., high MP-screening--test yields). However, the capacity of laboratory equipment and personnel for performing IDT and the availability of reagents are limited, and the higher false-positive rate of IDT (compared with MP screening) could have a negative short-term impact on the availability of blood in these regions. Approximately 4.5 million persons receive blood or blood products annually. Although persons needing blood transfusions should be aware of the limited risk for WNV infection, the benefits of receiving needed transfusions outweigh the potential risk for WNV infection. In addition, blood donation poses no risk to the donor for acquiring WNV, and the U.S. Public Health Service encourages blood donation. FDA, CDC, and the blood-collection community will continue to evaluate WNV-screening strategies to ensure blood safety. Acknowledgments This report is based in part on contributions by L Pietrelli, Roche Molecular Systems, Alameda, California. T Gahan, L DesJardin PhD, Univ of Iowa Hygienic Laboratory, Iowa City, Iowa. RS Lanciotti, PhD, A Lambert, A Noga, R Hochbein, Div of Vector-Borne Infectious Diseases, CDC. References
Figure 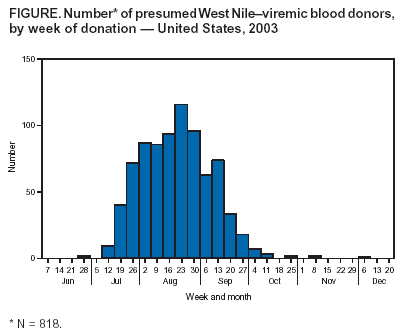 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/8/2004 |
|||||||||
This page last reviewed 4/8/2004
|