 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Cardiac Deaths After a Mass Smallpox Vaccination Campaign --- New York City, 1947During the first wave of the 2003 smallpox vaccination campaign, two ischemic cardiac deaths occurred in civilian vaccinees aged 55 and 57 years, and one occurred in a military vaccinee aged 55 years, 4--17 days after vaccination with the New York City Board of Health (NYCBOH) vaccinia strain (1--3). Whether these and 13 other recognized military and civilian nonfatal ischemic events among vaccinees were associated with smallpox vaccination is unclear. The same NYCBOH strain was used in 1947 to vaccinate approximately six million New York City (NYC) residents (80% of the population) during a 4-week period (April 4--May 2) after a smallpox outbreak (Figure 1). To determine whether smallpox vaccination increased the risk for cardiac death in 1947, the NYC Department of Health and Mental Hygiene (DOHMH) analyzed data from NYC death certificates during that period. This report summarizes the results of that analysis, which found no increases in cardiac, atherosclerotic, or all-cause deaths. The findings are consistent with a growing body of evidence suggesting that ischemic cardiac deaths observed after the 2003 campaign might have been unrelated to vaccine. In April 2003, data were extracted from NYC death certificates filed during March--June 1947 and from the same period in 1946 and 1948 (N = 81,529). DOHMH estimated the relative risk for cardiac deaths in the period after vaccination compared with other periods, adjusting for secular trends. The number of adults vaccinated on each of the 29 days of the vaccination campaign was estimated by using DOHMH records and articles from local newspapers and magazines (4). Death certificates issued in NYC during March--June in 1946--1948 were obtained from the NYC Municipal Archives. Date of death, age of decedent, and primary and other cause-of-death data (classified according to the International Classification of Diseases, Fifth Revision [ICD-5] codes) were abstracted from all records. Causes of death were defined as cardiac if the ICD-5 codes for primary or other cause included pericarditis (090), acute endocarditis (091), chronic endocarditis (092), myocardial disease (093), coronary artery diseases (094), or other disease of the heart (095). Certificates with illegible primary cause-of-death codes (0.6% of records) were excluded. Approximately 6.4 million NYC residents were vaccinated during April 4--May 2, 1947 (4) (Figure 2), including an estimated 500,000--1,000,000 persons each day during the peak 5 days of the vaccination campaign (April 17--21). The putative high-risk period for cardiac death was an estimated 4--17 days after vaccination, corresponding to the range of onset dates of cardiac events observed during the 2003 campaign. On the basis of these estimates, 2-week and 4-week risk periods were identified. Daily mortality rates during the postvaccination risk periods were compared with rates during other periods. Counts of cardiac deaths were modeled by using Poisson regression analysis, adjusting for a long-term temporal trend during 1946--1948 and a seasonal trend during March--June each year. Of the 81,010 legible records available, 39,150 (48%) listed cardiac disease and 9,112 (11%) specified coronary artery or atherosclerotic disease as a cause of death. Counts of cardiac deaths ranged from 72 to 149 deaths per day during the study period (Figure 3). The difference in the rate of cardiac deaths was not statistically significant during the 2-week risk period compared with other periods among persons aged 50--64 years (rate ratio: 1.05; 95% confidence interval [CI] = 0.95--1.15) or among all adults (rate ratio: 1.01; 95% CI = 0.95--1.07) (Table). Similarly, no statistically significant increases in risk were observed in all-cause deaths, atherosclerotic deaths, or deaths caused by myo/pericarditis during the 4-week risk period compared with other periods. Reported by: T Frieden, F Mostashari, SP Schwartz, New York City Dept of Health and Mental Hygiene, New York. LE Thorpe, Div of Adult Community Health, National Center for Chronic Disease Prevention and Health Promotion; AM Karpati, Epidemiology Program Office; MA Marx, SE Manning, EIS officers, CDC. Editorial Note:The findings in this report indicate that incidence of cardiac deaths did not increase after the 1947 mass smallpox vaccination campaign in NYC. The large number and proportion of persons vaccinated in a short time permitted a focused assessment of cardiac deaths after vaccination. These results suggest that cardiac deaths observed in 2003 might have been unrelated to smallpox vaccination. However, factors that could limit the applicability of the 1947 study results to the 2003 vaccination campaign include 1) changes in characteristics or administration of the vaccine, 2) changes in population distribution of cardiac risk factors, and 3) differences in the vaccination and smallpox infection history (i.e., immunity status) of vaccine recipients in the two periods. Both campaigns used the same NYCBOH vaccinia strain. Although long-term storage might have resulted in antigenic shift of the vaccine, DNA viruses such as vaccinia are not prone to antigenic variability (5). Both campaigns administered the vaccine intradermally. In 1947, vaccinators used various multiple-pressure techniques; the 2003 technique involved multiple punctures with a bifurcated needle to administer the vaccine. Both campaigns used a vaccine that contained a mixture of lymph and other components. Before 1960, the vaccine consisted of wet glycerinated lymph (with a titer of >106 plaque-forming units [pfu]/mL) composed of 50% glycerine and 50% calf lymph (6). Currently, lyophilized NYCBOH vaccinia containing calf lymph is mixed with a diluent containing polymixin B, streptomycin, chlortetracycline, and neomycin to a titer of >108 pfu/mL. However, no evidence has been found to indicate that these changes would lead to increases in cardiac adverse events after vaccination. Each of the 2003 vaccinees with cardiac fatalities had multiple risk factors for cardiac disease, including hypertension, hyperlipidemia, and smoking, and each had been vaccinated for smallpox in childhood. If risk factors for cardiac death were more prevalent in 2003 than in 1947, the number of cardiac-associated deaths probably would be greater among 2003 campaign vaccinees than among those in 1947. However, the prevalence of these three risk factors and cardiac mortality rates was substantially higher in 1947 than in 2003 (7,8). In addition, the 1947 vaccination campaign encouraged residents to participate regardless of health status, whereas the first wave of the 2003 campaign targeted only military, health-care, and emergency response professionals, all of whom were screened for noncardiac health problems and contraindications to vaccination. If a greater proportion of those vaccinated in 1947 were revaccinees compared with those vaccinated in 2003, and if previous vaccination reduced the risk for subsequent cardiac mortality, the 1947 findings would underestimate the risk for cardiac death after vaccination in 2003. However, nearly all of the 2003 civilian vaccinees were born before 1971, when childhood smallpox vaccination was routine in the United States, and would have received the smallpox vaccine once during childhood. This was an ecologic study; data about individual vaccination status for the 1947 population were unavailable. However, approximately 80% of the NYC population was vaccinated during the 1947 campaign. Although the 20% who were not vaccinated during the campaign might have differed systematically from the general population, any bias probably would not be substantial enough to alter the results of this study qualitatively. Myo/pericarditis after smallpox vaccination has been described previously (9) and has been observed in both civilians and military personnel vaccinated during the 2003 campaign. However, autopsy findings indicate that the 2003 cardiac deaths were linked not to myo/pericarditis but directly to ischemic events (2). In contrast to studies of inflammatory complications, few data support the association of ischemic cardiac adverse events with smallpox vaccination. Only one case series was found describing the experience of eight French vaccinees (of 12 million) aged 53--83 years who experienced acute ischemic events after smallpox vaccination, five of whom died (10). Smallpox vaccination is recommended for military personnel and civilian first responders without contraindications who are identified as part of terrorism preparedness and first-response teams. New screening guidelines have been instituted to minimize potential ischemic risks by excluding persons with known cardiac disease or three or more cardiac risk factors. Although this study casts doubt on the causal link between death caused by cardiac adverse events and smallpox vaccination, in the absence of a smallpox outbreak, all potential volunteers should be screened for risk factors, and those at high risk for adverse reactions to vaccination should be excluded. Acknowledgments This report is based on contributions by data entry staff, D Nash, New York City Dept of Health and Mental Hygiene; Public Health Library; Bur of Management Information Systems; Bur of Vital Statistics and Vital Records; New York City Dept of Records and Information Svcs; BS Chang, New York. DA Henderson, Johns Hopkins Bloomburg School of Public Health, Baltimore, Maryland. M Kulldorf, Div of Epidemiology and Biostatistics, Dept of Community Medicine and Health Care, Univ of Connecticut Health Center, Farmington, Connecticut. JM Lane, Atlanta, Georgia. J Grabenstein, U.S. Army Surgeon General's Office. Smallpox Vaccination and Adverse Events Team; National Immunization Program Cardiac Investigation Task Force, R Chen, S Chu, W Orenstein, National Immunization Program; D Stroup, J Livengood, Office of Science Policy and Technology Transfer; S Zaza, Epidemiology Program Office; L Neff, R Schreiber, Div of Adult Community Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. References
Figure 1  Return to top. Figure 2 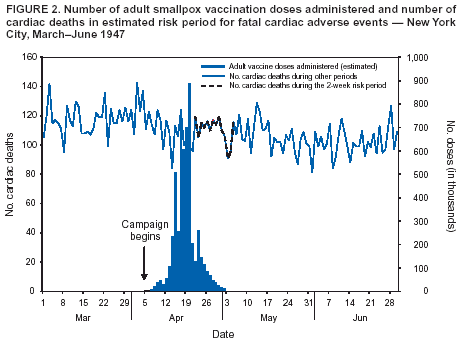 Return to top. Figure 3 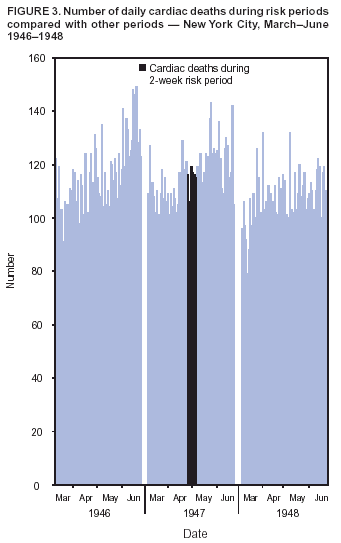 Return to top. Table 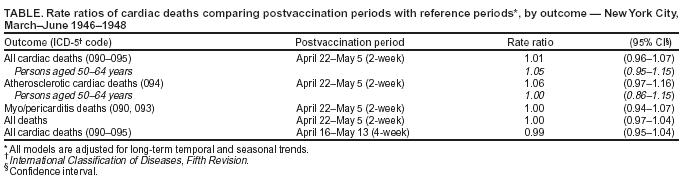 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/2/2003 |
|||||||||
This page last reviewed 10/2/2003
|