 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Cardiac and Other Adverse Events Following Civilian Smallpox Vaccination --- United States, 2003During January 24--June 20, 2003, smallpox vaccine was administered to 37,802 civilian health-care and public health workers in 55 jurisdictions to prepare the United States for a possible terrorist attack using smallpox virus. This report updates information on vaccine-associated adverse events among civilians vaccinated since the beginning of the program and among contacts of vaccinees, received by CDC from the Vaccine Adverse Event Reporting System (VAERS) as of June 20. Two cases of dilated cardiomyopathy (DCM) were diagnosed 3 months after vaccination. For the potential relation between smallpox vaccine and DCM to be assessed, identification of additional cases of DCM among vaccinees will be essential. Physicians who treat smallpox vaccine recipients are encouraged to evaluate and report patients with symptoms compatible with DCM, including those that occur several months after vaccination. In this vaccination program, CDC, the Food and Drug Administration, and state health departments are conducting surveillance for vaccine-associated adverse events among civilian vaccinees (1). As part of the vaccination program, civilian vaccinees receive routine follow-up, and persons reporting adverse events after vaccination receive follow-up as needed. The U.S. Department of Defense is conducting surveillance for vaccine-associated adverse events among military vaccinees and providing follow-up care to those persons with reported adverse events. Adverse events that have been associated with smallpox vaccination are classified on the basis of evidence supporting the reported diagnoses. Cases verified by virologic testing (or in some instances by other diagnostic testing) are classified as confirmed (Table 1). Cases are classified as probable if possible alternative etiologies are investigated and excluded and supportive information for the diagnosis is found. Cases are classified as suspected if they have clinical features compatible with the diagnosis, but either further investigation is required or investigation of the case did not provide supporting evidence for the diagnosis. All reports of events that follow vaccination (i.e., events associated temporally) are accepted; however, reported adverse events are not necessarily associated causally with vaccination, and some or all of these events might be coincidental. This report includes cases reported as of June 20 that either are under investigation or have a reported final diagnosis. Because discussions of final case definitions are ongoing, numbers and classifications of adverse events might change and will be updated regularly in MMWR. As of June 20, a total of 21 cases of myo/pericarditis were reported among civilians. Four of these were new suspected cases reported during May 10--June 20, including two cases of pericarditis and two cases of myocarditis (Table 1). In addition, eight cases of ischemic heart disease have been reported since the beginning of the civilian vaccination program, including five cases of myocardial infarction (MI) and three cases of angina. During May 10--June 20, one case of suspected generalized vaccinia was reported; no cases of eczema vaccinatum, erythema multiforme major, fetal vaccinia, or progressive vaccinia were reported (Table 1). In addition, 11 other serious adverse events were reported, including two cases of cardiomyopathy identified 3 months after smallpox vaccination in persons with no previous history of cardiomyopathy, coronary artery disease (CAD), or congestive heart failure. As of July 9, these cases were under investigation. Nine other serious events were reported, including three cases of chest pain, one case of gastro-esophageal reflux disease, one case of cholecystitis, one case of sudden death caused by atherosclerotic CAD 69 days postvaccination, and three neurologic cases were reported, including a central nervous system tumor diagnosed 28 days postvaccination, a headache evaluated for encephalitis, and a cerebral vascular accident. An additional 111 other nonserious events also were reported (Table 2). Among the 610 vaccinees with reported other nonserious adverse events during January 24--June 20, the most common signs and symptoms were fever (n = 121), rash (n = 114), headache (n = 103), pain (n = 95), and fatigue (n = 85) (Table 2). All of these commonly reported events are consistent with mild expected reactions following receipt of smallpox vaccine. Some vaccinees reported multiple signs and symptoms. During May 10--June 20, no vaccinia immune globulin was released for civilian vaccinees in the pre-event vaccination program, excluding persons involved in investigational new drug studies. No cases of vaccine transmission from civilian vaccinees to their contacts have been reported during the vaccination program (Table 3). A total of 14 cases of transmission from military personnel to civilian contacts have been reported since the program began. Case ReportsCase 1. On February 25, a woman aged 53 years with a history of untreated borderline hypertension and obesity was revaccinated; 7 days later, she had fatigue. On March 18, she continued to have fatigue and dyspnea, and she had symptoms of an upper respiratory infection and sinusitis for which she was prescribed antibiotics. On April 16, she saw her physician for an unrelated problem and was noted to have elevated blood pressure (150/100 mm/Hg). She was started on hydrochlorthiazide; her fatigue continued, and she had increasing exertional dyspnea. Other medications included postmenopausal hormone replacement therapy and antihistamines for seasonal allergies. She had no history of ischemic or valvular heart disease, autoimmune or metabolic disorders, excessive alcohol consumption, or exposure to other known cardiotoxic agents. On May 21, she had a routine scheduled physical examination performed by her regular physician. On cardiac examination, a murmur not detected previously was noted. An electrocardiogram (EKG) showed a left bundle branch block (LBBB), which was reported to be a new finding. On May 28, an echocardiogram showed normal left ventricular (LV) wall thickness, but mild dilatation with diffuse hypokinesis, moderate systolic function impairment, an ejection fraction (EF) of 35% (normal: >50%), and mild mitral regurgitation. On May 30, she reported to the emergency department with nonradiating, burning chest pain without dizziness, dyspnea, or palpitations. She was evaluated and had a cardiac catheterization, which showed no significant CAD but moderate global hypokinesis and EF of 35%. The findings were indicative of a nonischemic dilated cardiomyopathy. She began treatment with ramipril and metoprolol and has continued working. Case 2. On February 24, a woman aged 55 years with a history of obesity and moderately well-controlled hypertension, diabetes mellitus (DM), and hyperlipidemia was revaccinated. Nine days after vaccination, she had myalgias, arthralgias, and a temperature of 100º F (37.8º C) that resolved within 4 days; she reported no chest pain, dyspnea, or palpitations. On March 24, during a routine medical appointment, she reported continuing fatigue but no other symptoms. She had a family history of premature CAD but no history of angina, MI, congestive heart failure, autoimmune disease, excessive alcohol consumption, exposure to cardiotoxins, or metabolic disorders other than DM. Her medications included lisinopril, hydrochlorthiazide, atorvastatin, feofibrate, and metformin. On May 17, she had two brief episodes of palpitations, which did not recur. On June 3, she saw her physician for a routine appointment and complained of periodic fatigue since receiving her smallpox vaccination. On examination, she was noted to have a cardiac murmur not detected previously. On June 11, an EKG showed an LBBB that was not present on her most recent previous EKG in 1996. An echocardiogram showed moderate LV dilatation with spherical loss of architecture, moderate-to-severe symmetrical hypokinesis of all regional wall areas, severe depression of systolic function, and EF of 25%--30%. On June 24, an adenosine sestamibi stress test showed no evidence of ischemia, moderate LV enlargement, and EF of 23%, consistent with a nonischemic DCM. Her baseline medications were adjusted, and she has continued working. Reported by: Smallpox vaccine adverse events coordinators. National Immunization Program, CDC. Editorial Note:Cardiac adverse events including myocarditis and pericarditis have been reported following smallpox vaccination (2). Evidence suggests that myocarditis and pericarditis might be associated causally with vaccination (3). The two cases of DCM described in this report represent the first known temporal, although not necessarily causal, association of smallpox vaccination and DCM. However, whether vaccine caused these illnesses or whether the two cases were coincidental and would have occurred anyway is unclear. DCM is a syndrome characterized by cardiac enlargement and impaired systolic function of the left and/or right ventricle. Patients can have symptoms of congestive heart failure, syncope, arrhythmias, and systemic and pulmonary emboli; >40 causes of DCM have been described, including alcohol, toxins, infections, cytotoxic chemotherapy, and metabolic abnormalities (4--6). However, in approximately half of all cases, the cause is not identified; these are called idiopathic DCM (5,6). In a study of 673 patients admitted to a hospital with DCM, 81 (12%) had evidence of myocarditis on endomyocardial biopsy (5). Infectious causes of myocarditis include enteroviruses, adenoviruses, influenza, human immunodeficiency virus (HIV), and hepatitis C (4,6,7). Onset of viral-associated DCM can occur within 2--3 months of infection; however, many patients do not report previous viral symptoms or symptoms of myocarditis, and the time of onset of symptomatic DCM can be subtle and gradual. For these reasons, determining the timing of infection and attributing causality in many patients with viral-associated DCM is difficult. The mechanisms responsible for virus-related myocardial damage are not well understood; however, an autoimmune response is likely (6,7). Patients with DCM generally have echocardiograms that demonstrate dilated LV end-diastolic diameter and global or regional wall dyskinesia with poor LV contractile function (ejection fraction of <45%), often with some compensatory increased wall thickness. Viral studies might indicate infection (6). The use of endomyocardial biopsy in patients with probable DCM is not recommended routinely because <5% of patients have a condition for which a specific therapy is indicated (4--8). In one study, one fourth of patients reporting to a major medical center with symptomatic DCM died within a year, and half died within 5 years (6). Among those with myocarditis, survival rates are somewhat better (75% survival at 5 years and approximately 55% at 10 years) (9). Smallpox vaccination has not been associated previously with DCM. Because smallpox vaccination appears to be associated causally with myocarditis, which can cause DCM, further evaluation is warranted. DCM in either of the two cases described in this report could have been associated with other etiologies (e.g., preceding or interceding illnesses). As in other cases of DCM, attributing causality in these cases is difficult. The expected rate of DCM in this population is being calculated to determine if the observed rate of DCM among civilian vaccinees (two per 38,000) is higher than expected. Surveillance and follow-up are ongoing to identify additional cases of DCM among vaccinees. Guidelines for evaluation of possible DCM cases following vaccination are being developed. Clinicians who treat smallpox vaccinees should report patients with clinical presentations compatible with DCM to their state health department and to VAERS (1). References
Table 1 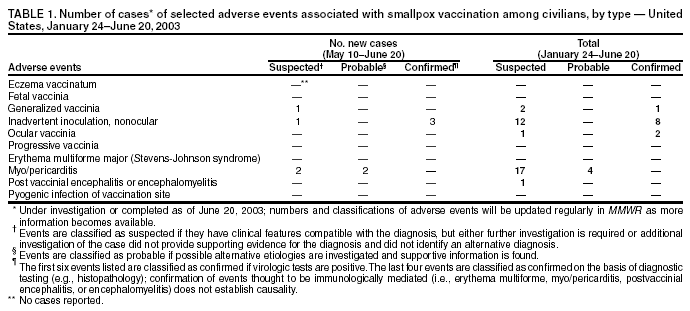 Return to top. Table 2 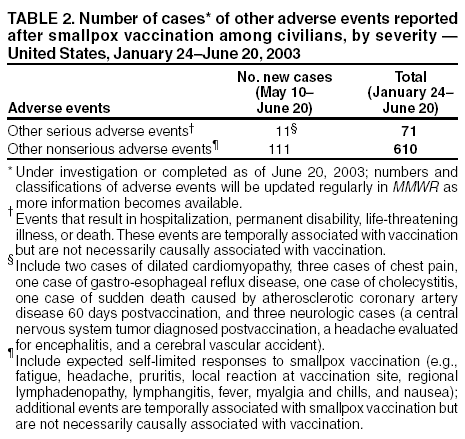 Return to top. Table 3 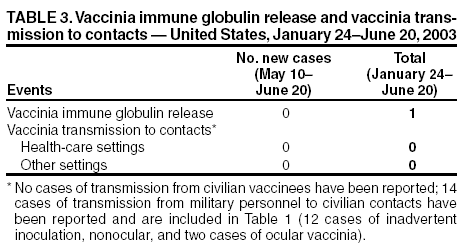 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/10/2003 |
|||||||||
This page last reviewed 7/10/2003
|