 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Imported Dengue --- United States, 1999 and 2000Dengue is a mosquito-transmitted acute viral illness caused by any of the four dengue virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4). Dengue is endemic in most tropical and subtropical areas of the world and has occurred among U.S. residents returning from travel to such areas. CDC maintains a laboratory-based passive surveillance system for imported dengue among U.S. residents (laboratory-diagnosed dengue in a U.S. resident living in an area without known authochthonous dengue transmission, with travel history outside the United States in the 14 days before symptom onset). The system relies on reports by clinicians to state health departments, which forward patient specimens to CDC for diagnostic testing. This report summarizes information about imported dengue cases among U.S. residents during 1999--2000. The findings indicate that dengue continues to cause disease in U.S. travelers abroad. Travelers to tropical areas should protect themselves from mosquito bites, and health-care providers should consider dengue in the differential diagnosis of illness for patients who have returned recently from such areas. Serum samples from 216 persons who had suspected dengue on the basis of clinical presentation and onset of symptoms in 1999 and 2000 were submitted to CDC from 34 states and the District of Columbia (1). From these samples, 41 (19%) cases were laboratory-diagnosed as dengue, of which 38 (93%) had IgM antibody or single high titers of IgG antibody in serum samples, and three (7%) patients had isolation of dengue virus (DEN-2, DEN-3, and DEN-4; one case each) (Table 1). Dengue diagnosis was negative in 112 (52%) patients, and indeterminate among 63 (29%) patients because convalescent samples for serologic testing were unavailable. Of the 40 laboratory-diagnosed dengue cases with available data, 22 (55%) were males. Age was reported for 35 persons (median: 37 years, range: 5--72 years). Clinical information was available for 28 patients with laboratory-diagnosed dengue. The most commonly reported symptoms were fever (100%), headache (64%), rash (54%), and myalgia (39%). At least three patients were identified as having been hospitalized, and one of these died (a male aged 41 years who had recently returned from Bangladesh). States reporting the highest number of cases were Massachusetts (four) in 1999 and New York (five) in 2000. Travel histories within the 2 weeks before illness, available for 33 persons, indicated that infections probably were acquired in Asia (13 cases), the Caribbean islands (12), Central America (seven), South America (one), and Africa (one). One patient reported traveling both in the Caribbean islands and South America. Data for both 1999 and 2000 indicated a marked decline in persons tested and in the percentage of persons laboratory-diagnosed with dengue, compared with 1997 and 1998, when 349 persons were tested and 143 (41%) were laboratory-diagnosed with dengue (2). Reported by: GG Clark, PhD, JG Rigau-Pérez, MD, V Vorndam, PhD, Div of Vector-Borne Infectious Diseases, National Center for Infectious Diseases; JM Hayes, DrPH, EIS Officer, CDC. Editorial Note:Dengue is transmitted to humans by Aedes mosquitoes. The majority of U.S. residents with dengue become infected during travel to tropical areas, although autochthonous transmission of dengue was documented in Texas in 1999 (3,4), and Hawaii in 2001 (5). The 1999 Texas outbreak was limited in scope because environmental factors (e.g., air conditioning) limited contact with Ae. aegypti mosquitoes. In Hawaii, the outbreak might have been limited because the vector was Ae. albopictus, a less efficient vector for dengue than Ae. aegypti, which was not detected in localities where dengue transmission occurred. The incubation period of dengue has a range of 3--14 days (in the majority of cases, 4--7 days). Dengue virus infection can be asymptomatic or cause illnesses ranging from mild undifferentiated fever to severe disease, including hemorrhagic manifestations and shock (6). Dengue hemorrhagic fever (DHF) is characterized by fever, minor or major bleeding phenomena, thrombocytopenia (<100,000 platelets/mm3), and evidence of increased vascular permeability (e.g., hemoconcentration [hematocrit increased by >20% from baseline], pleural or abdominal effusions, or hypoproteinemia) (6). Dengue shock syndrome (DSS) is DHF with signs of circulatory failure, including narrow pulse pressure (<20 mmHg), hypotension, or shock, and might result in a case fatality rate of approximately 10% (7). During 1993--1998, the number of imported laboratory-diagnosed U.S. cases increased, reflecting the impact of travel and the occurrence of epidemic dengue in 1995 and 1998, especially in the Caribbean and Central America. The smaller number of laboratory-diagnosed cases of dengue in 1999 and 2000 is temporally associated with a decreased number of cases of dengue/DHF in the Americas (8). The findings in this report are subject to at least two limitations. First, travel histories and clinical information were available only for certain persons with dengue, and they might not be representative of all persons with imported dengue. Second, the number of dengue cases referred to CDC for diagnosis represents a minimum estimate of the actual number of U.S. travelers with dengue fever or DHF/DSS. Diagnostic samples might not have been sent for testing or might have been sent to other laboratories. Because dengue is not nationally reportable, persons with suspected dengue might not be reported. For example, in response to the 1999 documented authochthonous transmission of dengue, Texas implemented a state laboratory-based active surveillance system. Of the 462 patients whose specimens were submitted for testing at the Texas Department of Health (TDH) or other reference laboratories that year, 66 (14.3%) were laboratory-diagnosed as dengue (TDH, surveillance data, cases not included in this report). Persons traveling to areas where dengue is endemic should avoid exposure to mosquitoes by using repellents, wearing protective clothing, and remaining in well-screened or air-conditioned areas. No vaccine is available for preventing dengue infection. Health-care providers should consider dengue in the differential diagnosis of illness for all patients who have fever and a history of travel to tropical areas within 2 weeks before the onset of symptoms. Supportive measures should be administered, and only acetaminophen is recommended for management of pain and fever. Acetylsalicylic acid (i.e., aspirin) and other nonsteroidal anti-inflammatory agents are contraindicated because of their anticoagulant properties. Dengue patients should be monitored for signs of DHF, especially hypotension, because prompt fluid therapy reduces morbidity and mortality. Acute-phase (0--5 days after onset of symptoms) and convalescent-phase (6--30 days after onset of symptoms) serum samples obtained for viral isolation and diagnosis should be sent through state or territorial health departments to CDC's Dengue Branch, Division of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, 1324 Calle Cañada, San Juan, PR 00920-3860, telephone 787-706-2399, fax 787-706-2496. Serum samples should be accompanied by a summary of clinical and epidemiologic information, including date of onset of disease, date of collection of sample, and a detailed recent travel history. AcknowledgmentThis report is based on data contributed by state health departments. References
Table 1 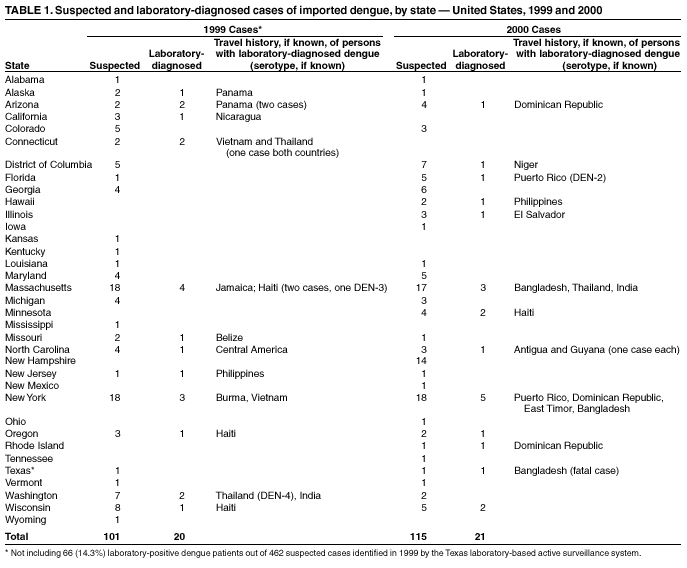 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/4/2002 |
|||||||||
This page last reviewed 4/4/2002
|