 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Influenza Activity --- United States and Worldwide, May--September 2001During October 2000--May 2001, influenza A (H1N1), A (H3N2), and B viruses were identified in the Northern Hemisphere. Influenza A (H1N1) and B viruses circulated widely; influenza A (H3N2) viruses were reported infrequently and were not associated with widespread outbreaks in any country during October 2000--May 2001. Since May 2001, influenza A (H1N1) and B viruses have predominated in Asia and Oceania; influenza A (H3N2) and B viruses have predominated in Africa and South America. This report summarizes influenza activity in the United States* (1) and worldwide during May--September 25; influenza A (H1N1), A (H3N2), and B viruses continued to circulate worldwide and were associated with mild to moderate levels of activity. This activity underscores the need to follow the recommendations of the Advisory Committee on Immunization Practices (ACIP) (2,3) for the timely vaccination of persons at high risk for influenza-related complications. United StatesInfluenza B viruses were reported more frequently than influenza A viruses during May 2001. Unsubtyped influenza A viruses were reported from Alaska and Missouri during June; influenza A (H1N1) viruses were reported from one case in Michigan and one case in Texas in June and July, respectively. In Hawaii, an influenza A (H3N2) virus was isolated from one case during June and two A (H1N1) viruses were isolated in July and August. Influenza B viruses were isolated in Hawaii each month during May-- July. WorldwideDuring May--September 25, influenza A (H1N1) and B viruses circulated in Asia and Oceania; influenza A (H3N2) viruses were identified less frequently than influenza A (H1N1) and B viruses and were not associated with widespread activity. In Africa, influenza A (H3N2) viruses were reported from Senegal and South Africa, and influenza B viruses were reported from Mauritius and South Africa. In South America, influenza A (H3N2) and B viruses circulated widely, and influenza A (H1N1) viruses have been identified less frequently. Influenza A (H3N2) viruses predominated in Argentina and Chile; in Brazil and Paraguay, influenza type B viruses were reported more frequently than influenza type A viruses. Both influenza A (H3N2) and B viruses also were reported from Uruguay. In Canada, influenza A and B viruses were identified during May--August. Isolate AnalysisThe WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza at CDC analyzes isolates from laboratories worldwide. This report describes the antigenic characteristics of influenza isolates collected during May--September 25, including isolates from the Northern Hemisphere and Southern Hemisphere. Of the 25 influenza A (H1N1) isolates antigenically characterized, 24 (96%) were similar to A/New Caledonia/20/99, the H1N1 component of the 2001--02 influenza vaccine, and one (4%) showed reduced titers with A/New Caledonia/20/99 antisera. Of the 25 influenza A (H1N1) isolates, six were from Asia, 10 were from Oceania, eight were from South America, and one was from the United States. Of the 67 influenza A (H3N2) viruses antigenically characterized, 61 (91%) were similar to A/Panama/2007/99, the H3N2 component of the 2001--02 influenza vaccine, and six (9%) showed reduced titers with A/Panama/2007/99 antisera. Of the 67 influenza A (H3N2) viruses, 17 were from Asia, two were from Oceania, 47 were from South America, and one was from the United States. Circulating influenza B viruses can be divided into two antigenic and genetic groups represented by B/Yamagata/16/88 and B/Victoria/2/87 reference strains (Table 1). B/Victoria/2/87 group viruses circulated widely before 1991; however, during 1991--2000, these viruses were identified only in Asia (China, Hong Kong/China, Japan, Singapore, Taiwan, and Thailand). Since 1990, B/Yamagata/16/88 group viruses have circulated worldwide. The recommended influenza B vaccine strain, B/Sichuan/379/99, belongs to the B/Yamagata/16/88 group. Most of the viruses in the B/Victoria/2/87 group are represented by the reference strain B/Hong Kong/22/2001. Of the 54 antigenically characterized influenza B viruses collected worldwide during May--September 25, 37 (69%) belong to the B/Yamagata/16/88 group, and 17 (31%) belong to the B/Victoria/2/87 group. Of 37 B/Yamagata/16/88 group viruses, 35 (95%) were similar to recommended vaccine strain B/Sichuan/379/99, and two (5%) showed reduced titers with B/Sichuan/379/99 antisera. These B/Sichuan/379/99-like viruses were from the United States (Hawaii and Massachusetts), Asia (China, Hong Kong/China, Japan, and Thailand), South America (Argentina, Brazil, Chile, and Costa Rica) and Oceania (Australia and New Zealand). The influenza B/Victoria/2/87 group viruses collected during May--September 25 were isolated in Hawaii and Hong Kong/China. During March, one B/Victoria/2/87 group virus was reported in Canada from a patient who recently had traveled in Asia. No B/Victoria/2/87 group viruses have been identified in Europe, Oceania, or South America. Reported by: World Health Organization National Influenza Centers, Communicable Diseases, Surveillance and Response, World Health Organization, Geneva, Switzerland. A Hay, PhD, WHO Collaborating Center for Reference and Research on Influenza, National Institute for Medical Research, London, England. I Gust, MD, A Hampson, WHO Collaborating Center for Reference and Research on Influenza, Parkville, Australia. M Tashiro, MD, WHO Collaborating Center for Reference and Research on Influenza, National Institute of Infectious Diseases, Tokyo, Japan. R Ueki, S Nagaue, L Muus, G Kunimoto, Virology Section, State Laboratories Div; T Tom, MS, M Ieong, MPH, Epidemiology Br, Hawaii Dept of Health, Honolulu. WHO collaborating laboratories. National Respiratory and Enteric Virus Surveillance System laboratories. WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza, Influenza Br and Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial Note:Influenza A (H1N1), A (H3N2), and B viruses circulated during the winter in the Southern Hemisphere (May--mid-September) and summer (May--mid-September) in the Northern Hemisphere. The identification of influenza cases and sporadic influenza outbreaks during summer and fall are not unusual. From June through mid-September, few influenza isolates were identified in Alaska, Canada, and the continental United States. Most of the viruses identified were influenza type A; two influenza A viruses were subtyped (H1N1). The most frequently isolated influenza viruses in Hawaii were influenza B viruses and both of the antigenic groups have been identified. Most of the viruses isolated worldwide since May are well matched to the current vaccine strains. For the 2001--02 season in the Northern Hemisphere, the type(s) and subtype(s) of influenza virus that will circulate and the onset, peak, and severity of disease activity cannot be predicted. The optimal time for persons at increased risk for influenza-related complications to receive annual influenza vaccination is October and November; vaccination of other persons should continue through December and later as long as vaccine is available (2,3). The three influenza vaccine manufacturers distributing in the United States are expected to produce and distribute approximately 79 million doses combined. Distribution of 44.6 million doses (56% of projected season totals) may be delayed until the end of October; the remaining 35 million doses are expected to be distributed in November and December. During July 2001, ACIP issued recommendations to address an anticipated delay in influenza vaccine availability (3). The primary recommendations for health-care providers were 1) to target vaccine available in September and October to persons at increased risk for influenza complications and to health-care workers, 2) beginning in November, also to offer vaccine to contacts of high-risk persons, healthy persons aged 50--64 years, and others who want to reduce their risk for influenza, and 3) to continue vaccinating patients, especially those at high risk and in other target groups, in December and throughout the influenza season as long as vaccine is available. Recommendations for influenza vaccine manufacturers and distributors were 1) to delay until November distribution of vaccine to worksites, where campaigns primarily vaccinate healthy persons, and 2) to process orders so that all providers who have ordered vaccine receive some early season vaccine. Previously, ACIP extended the recommended optimal time for vaccination from October through the end of November (2). Each February, the World Health Organization (WHO) recommends influenza virus strains for inclusion in the following season's Northern Hemisphere influenza vaccine (4). The regulatory authorities in each country then determine the actual viruses to be used for vaccine production. In the United States, the Food and Drug Administration's Vaccines and Related Biological Products Advisory Committee is responsible for the selection of vaccine strains to be used by U.S. vaccine manufacturers. The regulatory authorities in a country frequently will substitute an antigenically equivalent virus for one or more of the WHO recommended viruses because of better growth or processing properties. For the 2001--02 influenza season, WHO recommended A/New Caledonia/20/99-like (H1N1), A/Moscow/10/99-like (H3N2), and B/Sichuan/379/99-like viruses for inclusion in the Northern Hemisphere influenza vaccine (4). Influenza vaccines sold in North America will use A/New Caledonia/20/99 for the H1N1 component and the antigenically equivalent stains of A/Panama/2007/99 (H3N2) for the A/Moscow/10/99-like strain and B/Johannesburg/5/99, B/Victoria/504/2000, or B/Guangdong/120/2000 for the B/Sichuan/379/99-like strain. Updated information about vaccine availability is available at <http://www.cdc.gov/nip/flu/>. This site also has patient education materials, suggestions on establishing a patient reminder/recall system, and other information that may assist providers in distributing influenza vaccine. Formal surveillance for influenza in the United States and reporting by CDC is conducted during October--May. This information is updated weekly and is available through CDC's voice information system, telephone (888) 232-3228, the fax information system, telephone (888) 232-3299, by requesting document number 361100, or at <http://www.cdc.gov/ncidod/diseases/flu/weekly.htm>. References
*The four components of the influenza surveillance system have been described (1).
Table 1 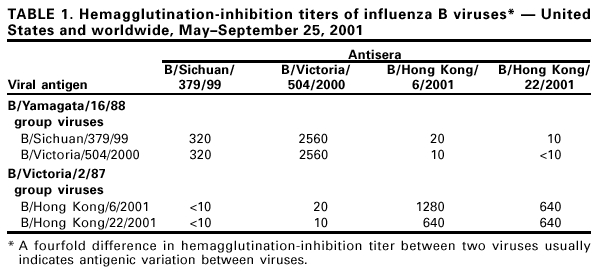 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/28/2001 |
|||||||||
This page last reviewed 9/28/2001
|