 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Trends in Screening for Colorectal Cancer --- United States, 1997 and 1999Colorectal cancer is the second leading cause of cancer-related death in the United States (1). An estimated 135,400 new cases and 56,700 deaths from colorectal cancer are expected during 2001 (1). Since the mid-1990s, national guidelines have recommended that persons aged >50 years at average risk for colorectal cancer should have screening tests regularly. To estimate rates for the use of colorectal cancer screening tests and to evaluate trends in test use, CDC analyzed data from the 1999 Behavioral Risk Factor Surveillance System (BRFSS) on the use of a home administered fecal occult blood test (FOBT) and sigmoidoscopy/colonoscopy, and then compared them with similar data from 1997. The findings in this report indicate that the proportion of the U.S. population that has been screened remains low. In 1999, 44% of BRFSS respondents reported receiving FOBT and/or sigmoidoscopy/colonoscopy within the recommended period compared with approximately 41% reporting FOBT and/or sigmoidoscopy/proctoscopy within the recommended period in 1997 (2). Efforts to address barriers and to promote the use of colorectal cancer screening should be intensified. In 1999, the 50 states, District of Columbia, and Puerto Rico participated in BRFSS, an ongoing, statebased, randomdigit--dialed telephone survey of the civilian, noninstitutionalized population aged >18 years. A total of 63,555 respondents aged >50 years were asked whether they ever had FOBT using a home kit, whether they ever had sigmoidoscopy or colonoscopy, and when the last test had been performed. Responses coded as "don't know/not sure" or "refused" were excluded from analyses (<2%). Aggregated and state-specific proportions, standard errors, 95% confidence intervals, and p-values were calculated using SAS and SUDAAN. Data in this analysis were weighted to the age, sex, and race/ethnicity distribution of each state's adult population using intercensal estimates and were age standardized to the 1999 BRFSS population. The median state response rate of 56.7% (range: 38.4%--83.9%) was calculated using the cooperation rate formula (i.e., the number of completed interviews divided by the number of potential respondents [households with a resident aged >18 years]). The 1999 questions about the use of sigmoidoscopy were modified from the 1997 questions. In 1997, respondents were asked whether they had received sigmoidoscopy or proctoscopy. Proctoscopy is performed with a shorter instrument than sigmoidoscope and is not recommended as a colorectal cancer screening test. In 1999, "sigmoidoscopy/proctoscopy" was replaced with "sigmoidoscopy/colonoscopy." Colonoscopy evaluates the entire colon and is recommended once every 10 years in some guidelines (3,4). For this report, "sigmoidoscopy/proctoscopy" and "sigmoidoscopy/colonoscopy" are referred to as "sigmoidoscopy" unless otherwise specified. In 1999, 40.3% (25,263 of approximately 63,000) of respondents reported ever having FOBT, and 43.8% (26,388) of the respondents reported ever having sigmoidoscopy. For tests received within the recommended period, 20.6% (12,518) had FOBT within the year preceding the survey, 33.6% (19,535) had sigmoidoscopy within the preceding 5 years (Table 1), and 44.0% (25,871) had either FOBT within the year preceding the survey or sigmoidoscopy within the preceding 5 years (Figure 1). In 1997, 19.6% (9832 of approximately 51,000) of the respondents had FOBT within the year preceding the survey, and 30.3% (14,678) had sigmoidoscopy within the preceding 5 years (Table 1). Although these rate changes in testing use were statistically significant (p<0.05), actual increases were small. By state, the proportion of respondents who had FOBT within the preceding year ranged from 8.2% (112 of 1366) in Puerto Rico to 36.4% (187 of 500) in the District of Columbia; the proportion that had sigmoidoscopy/colonoscopy within the preceding 5 years ranged from 20.4% (275 of 1357) in Puerto Rico to 46.1% (410 of 981) in Delaware (Table 2). Reported by the following state BRFSS coordinators: S Reese, MPH, Alabama; P Owen, Alaska; B Bender, MBA, Arizona; G Potts, MBA, Arkansas; B Davis, PhD, California; M Leff, MSPH, Colorado; M Adams, MPH, Connecticut; F Breukelman, Delaware; I Bullo, District of Columbia; S Hoecherl, Florida; L Martin, MS, Georgia; F Reyes-Salvail, MS, Hawaii; J Aydelotte, MA, Idaho; B Steiner, MS, Illinois; L Stemnock, Indiana; J Davila, Iowa; C Hunt, Kansas; T Sparks, Kentucky; B Bates, MSPH, Louisiana; D Maines, Maine; A Weinstein, MA, Maryland; D Brooks, MPH, Massachusetts; H McGee, MPH, Michigan; N Salem, PhD, Minnesota; D Johnson, MS, Mississippi; J Jackson-Thompson, PhD, Missouri; P Feigley, PhD, Montana; L Andelt, PhD, Nebraska; E DeJan, MPH, Nevada; L Powers, MA, New Hampshire; G Boeselager, MS, New Jersey; W Honey, MPH, New Mexico; C Baker, New York; Z Gizlice, PhD, North Carolina; L Shireley, MPH, North Dakota; P Pullen, Ohio; K Baker, MPH, Oklahoma; K Pickle, MPH, Oregon; L Mann, Pennsylvania; Y Cintron, MPH, Puerto Rico; J Hesser, PhD, Rhode Island; M Wu, MD, South Carolina; M Gildemaster, South Dakota; D Ridings, Tennessee; K Condon, MS, Texas; K Marti, Utah; C Roe, MS, Vermont; K Carswell, MPH, Virginia; K Wynkoop Simmons, PhD, Washington; F King, West Virginia; K Pearson, Wisconsin; M Futa, MA, Wyoming. Epidemiology and Health Svcs Research Br, Div of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, CDC. Editorial Note:Since 1997, the proportion of the U.S. population that reported having had FOBT and sigmoidoscopy has increased slightly but remains low. Various factors may contribute to the continued underuse of these tests, including lack of knowledge by the public and health-care providers of the effectiveness of screening and low reimbursement rates for health-care providers who perform screening tests (5,6). The findings in this report are subject to at least four limitations. First, because of the wording change in the BRFSS questionnaire from "sigmoidoscopy/proctoscopy" in 1997 to "sigmoidoscopy/colonoscopy" in 1999, comparing endoscopic procedures for these years must be interpreted with caution. Data on the use of colonoscopy were collected only in 1999; however, some tests reported as sigmoidoscopies/proctoscopies in 1997 probably were colonoscopies because some respondents may have been unable to distinguish among the three tests. It is unknown whether the reported increase from 1997 to 1999 represents a true increase in sigmoidoscopy use or previously unmeasured rates of colonoscopy use. Second, because the survey was administered over the telephone, only persons who own telephones were represented in this analysis. Third, 43.3% of the eligible respondents were contacted but did not complete the telephone interview or could not be reached for an interview. Finally, responses were self-reported and were not validated through medical record review. For persons aged >50 years at average risk for colorectal cancer, recommended screening options include one or more of the following tests: annual FOBT, sigmoidoscopy every 5 years, colonoscopy every 10 years, or double-contrast barium enema every 5--10 years (3,4,7). Despite their efficacy in reducing incidence and mortality from colorectal cancer (8), screening tests are underused. To draw attention to this disease, the U.S. Congress designated March as "National Colorectal Cancer Awareness Month." During March 2001, CDC and the Health Care Financing Administration launched the third annual "Screen for Life: A National Colorectal Cancer Action Campaign." Using print, television, and radio announcements and brochures and fact sheets, the campaign was designed to raise awareness of colorectal cancer and to encourage persons aged >50 years to discuss screening with their health-care provider and select the appropriate test(s). CDC also produced "A Call to Action: Prevention and Early Detection of Colorectal Cancer," a slide presentation for health-care providers. All material is available on the World-Wide Web, http://www.cdc.gov/cancer/screenforlife and http://www.cdc.gov/ cancer/colorctl/calltoaction. References
Table 1 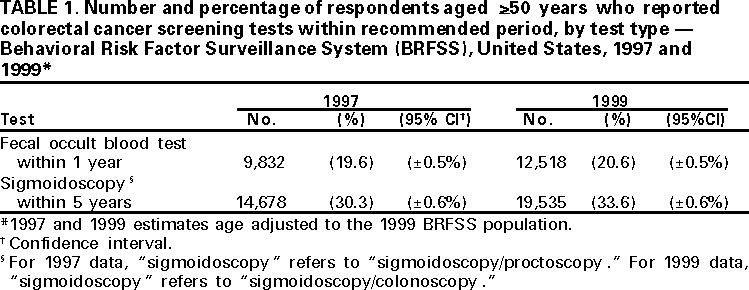 Return to top. Figure 1 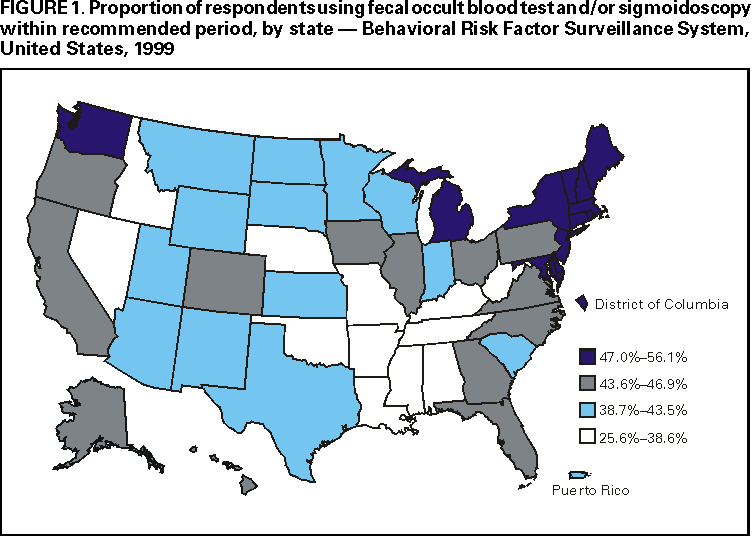 Return to top. Table 2 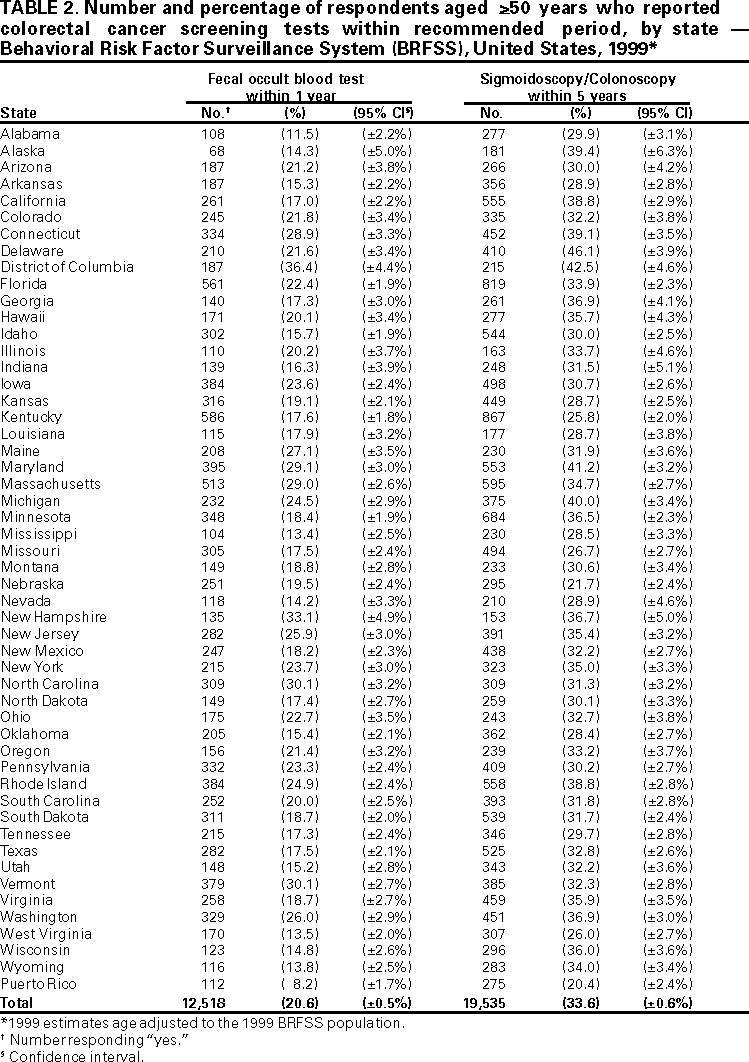 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/8/2001 |
|||||||||
This page last reviewed 5/2/01
|