 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Measles, Rubella, and Congenital Rubella Syndrome --- United States and Mexico, 1997--1999Please note: An erratum has been published for this article. To view the erratum, please click here. In 1996, the Immunization Working Group of the Mexico-United States Binational Commission was established to enhance coordination of disease surveillance, assure high vaccination coverage in both countries, and hasten the elimination of vaccine-preventable diseases. The United States and Mexico share the Pan American Health Organization (PAHO) goal of measles elimination by 2000 (1). The United States also established a goal of eliminating indigenous rubella and congenital rubella syndrome (CRS) by 2000 (2). This report summarizes the measles and rubella vaccination and surveillance data for the United States and Mexico for 1997--1999. Measles in the United StatesMeasles epidemiology in the United States is monitored through the National Notifiable Diseases Surveillance System (NNDSS). Record low numbers of measles cases were reported in the United States for 1997 (138 cases), 1998 (100), and 1999 (100), corresponding to 0.5 cases per 100,000 population (Figure 1). Among these 338 cases, 116 (34%) were imported from other countries, 63 (19%) were epidemiologically linked to imported cases, and 39 (12%) showed virologic evidence of importation. The remaining 120 cases (36%) were not attributed to importation. None of the 338 cases reported during 1997--1999 was imported from Mexico. Surveillance quality indicators were implemented in 1996. In March 1999, a panel of experts concluded that measles was no longer endemic in the United States (3). Measles vaccination levels among children aged 2 years increased from 61% in 1985 (CDC, unpublished data, 1998) to 91% in 1997 (4). As of the 1998--99 school year, state laws requiring a second dose for students in grades K-12 applied to 60% of U.S. students (CDC, unpublished data, 2000). Measles in MexicoMeasles epidemiology in Mexico was monitored through the Single Epidemiological Surveillance System (SUIVE) until 1993, when the Febrile Exanthematic Disease Surveillance System (FEDSS) was established to incorporate laboratory information to distinguish among viral causes of rash illnesses. During 1997--1999, no confirmed cases of measles were reported (5). National surveillance indicator goals to evaluate the quality of FEDSS were established in 1993, and by 1999, most goals had been met. After 1990, when 68,782 cases (80 per 100,000) and 5,899 deaths were attributed to measles (6), multiple strategies have resulted in high vaccination coverage in children (Figure 1). In May 1998, the National Immunization Council replaced measles-only childhood vaccination with measles-mumps-rubella (MMR) vaccine, moving the first dose from 9 to 12 months and keeping the second dose at age 6 years. National Health Weeks are conducted three times a year, during which unvaccinated preschool and first-grade children are vaccinated. During 1997, among children aged 1 to 4 years, first-dose coverage was 97%, a level that was maintained during 1998--1999. Rubella and CRS in the United StatesRubella and CRS incidence is monitored through NNDSS and the National Congenital Rubella Syndrome Registry. Rubella vaccine was licensed in 1969, and since 1979, has been administered in combination as MMR; rubella coverage closely approximates measles coverage. In the United States in 1997, 1998, and 1999, 172, 353, and 267 confirmed cases of rubella were reported, respectively, corresponding to <0.5 cases per 100,000 population (Figure 2). Most of these cases occurred among Hispanic men. Of the 788 cases for whom age was known, 676 (80.4%) were aged 15--44 years. Of the 790 case-patients for which sex was known, 507 (64.0%) were men. Of the 755 for whom ethnicity was reported, 587 (77.7%) were Hispanic; the percentage of reported rubella cases among Hispanics increased from 19.0% in 1991 to 77.6% in 1999. Since 1998, of the 340 outbreak-related cases with known country of origin, 273 (80.0%) occurred among persons who were non-U.S. born. Of the 661 cases for which importation status was known, 54 (8.2%) were internationally imported; of these, exposures occurred in Mexico, Central and South America, the Spanish-speaking Caribbean, Japan, and Russia. Of 24 infants with laboratory-confirmed CRS born during 1997--1999, 20 (83.3%) were born to Hispanic mothers, 14 (58.3%) were born to non-U.S.--born mothers, and 10 (41.7%) had maternal exposure to rubella outside the United States and were considered imported cases. Rubella and CRS in MexicoRubella epidemiology in Mexico has been monitored since 1978 as clinically diagnosed cases reported to SUIVE or, since 1993, as laboratory-confirmed cases evaluated by FEDSS; once confirmed as rubella, FEDSS also followed women infected during pregnancy to detect potential cases of CRS. In 1998, rubella vaccine was introduced into the childhood vaccination schedule as 2-dose MMR at age 1 and 6 years. From 1978 through 1999, reported rubella cases peaked every 3--5 years, with the highest number of cases (65,591; rate: 79 per 100,000 population) reported in 1990. From 1997 to 1999, 38,042; 51,846; and 21,173 rubella cases, respectively, were reported to SUIVE (Figure 2). Compared with 1990, in 1999, reported rubella cases decreased 68%. During 1997--1999, 37,346 (33.6%) of the reported case-patients were aged 15--44 years. Of the 4650 cases of rash illness investigated by FEDSS during this time, 3277 (70.5%) were classified as rubella, and 1373 (29.5%) were classified as other rash illnesses. Surveillance among 266 pregnant women infected during rubella outbreaks from 1997 to 1999 detected 50 confirmed cases of CRS. Reported by: E Ferreira, MD, V Carrión, MD, CA Lucas, DDS, P Kuri, MD, Office of the General Directorate of Epidemiology; A Flisser, DrSc, National Reference Laboratory; JL Diaz Ortega, MD, JIS Preciado, MD, National Immunization Council; RT Conyer, MD, Office of the Under Secretary for Disease Control and Prevention, Ministry of Health, Mexico. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Child Vaccine Preventable Diseases Br, Epidemiology and Surveillance Div, National Immunization Program; and an EIS Officer, CDC. Editorial Note:Since the measles epidemic during 1989--1991, substantial progress has been made in vaccination programs in Mexico and the United States, as evidenced by the control of measles in both countries. Mexico reported no cases during 1997--1999, despite enhanced surveillance for measles that includes investigating >1500 suspected cases each year. In the United States, the low number of reported cases, the preponderance of importation-related cases, the geographic isolation of each case, and the lack of a recurring viral measles strain indicate that measles is no longer endemic in the United States (3). The consistent detection of imported measles cases is evidence of the sensitivity of U.S. measles surveillance. The benefit of concurrent improvements in measles control is demonstrated by the absence of imported cases from Mexico into the United States during 1997--1999. The United States is on the verge of eliminating indigenous rubella and CRS. However, rubella outbreaks continue to occur, primarily among Hispanics from countries where no national routine rubella vaccination program exists or where a program has been implemented only recently. Because universal rubella vaccination in Mexico was introduced in 1998, ongoing rubella and CRS surveillance will be important to document the impact of the new program. After successfully implementing measles-rubella (MR) vaccination among health-care personnel, Mexico implemented MR vaccination campaigns among at-risk adolescents and adults, including junior and senior high school students and teachers in October 2000. Mass vaccination of adolescents and adults will accelerate the decline in rubella and CRS cases and prevent the re-entry of measles. Measles remains a leading cause of morbidity and mortality worldwide. The United States and Mexico have achieved the PAHO goal of eliminating endemic transmission of measles. For countries undertaking measles elimination, integrating rubella control into measles elimination activities is a preferred strategy because of the similar surveillance activities and intervention target groups for MR/MMR vaccine (7). In countries where the health burden from rubella has been documented and where immunity among women of childbearing age can be assured, implementing a universal childhood rubella vaccination program with >80% coverage will lead to a decline in rubella and CRS (7). References
Figure 1 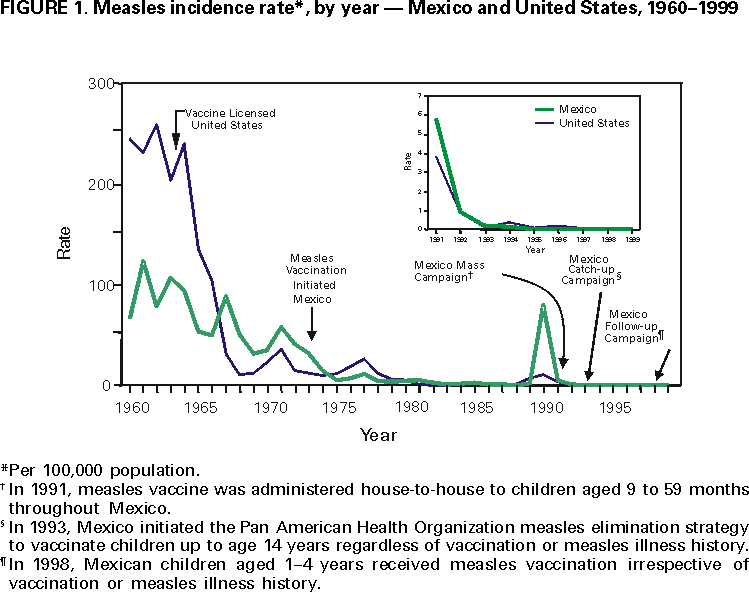 Return to top. Figure 2 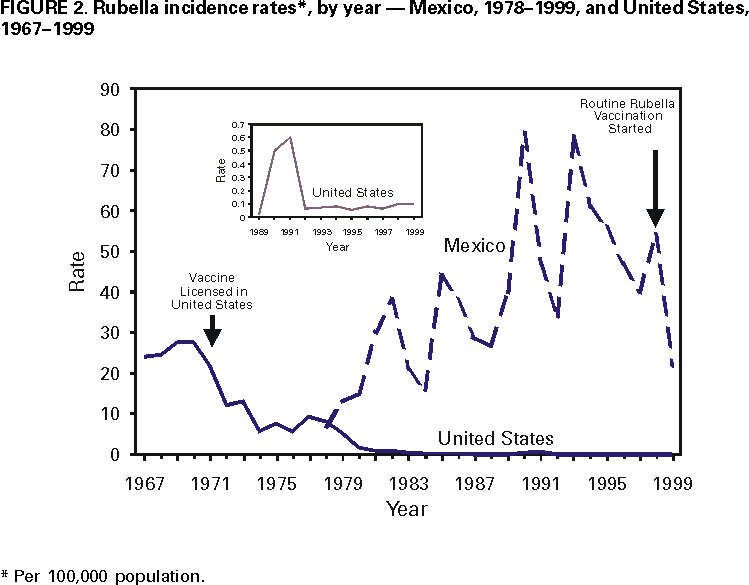 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/21/2000 |
|||||||||
This page last reviewed 5/2/01
|