 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Fluoroquinolone-Resistance in Neisseria gonorrhoeae, Hawaii, 1999, and Decreased Susceptibility to Azithromycin in N. gonorrhoeae, Missouri, 1999In 1999, 360,076 cases of gonorrhea were reported in the United States (1). Gonorrhea is a major cause of pelvic inflammatory disease, often leading to ectopic pregnancy and infertility, and it can facilitate human immunodeficiency virus (HIV) transmission (2). During the 1980s, resistance to penicillin and tetracycline among gonococcal isolates became widespread; as a result, CDC recommended that other antimicrobial agents be used to treat gonorrhea. This report summarizes investigations of an increase in fluoroquinolone-resistant Neisseria gonorrhoeae in Hawaii and of a cluster of N. gonorrhoeae infections with decreased susceptibility to azithromycin in Missouri. N. gonorrhoeae with fluoroquinolone-resistance, HawaiiThe susceptibility of N. gonorrhoeae to ciprofloxacin is used to assess susceptibility to all equivalent fluoroquinolone antimicrobials. The Hawaii Department of Health State Laboratory (HSL) routinely performs antimicrobial susceptibility testing on all gonococcal isolates identified by culture. HSL also submits gonococcal isolates from the Diamond Head Health Center STD and HIV Clinic in Honolulu, Hawaii, to the Gonococcal Isolate Surveillance Project (GISP), a CDC-sponsored sentinel surveillance system that monitors antimicrobial resistance of N. gonorrhoeae. The 26 sexually transmitted disease (STD) clinics in the United States that participate in GISP collect male urethral gonococcal cultures and submit them to one of five regional GISP laboratories for antimicrobial susceptibility testing. An increase in the number of ciprofloxacin-resistant (CipR)* gonococcal isolates submitted by HSL to CDC for reference characterization in 1999 (3) prompted CDC and the Hawaii Department of Health (HDH) to initiate an investigation in September 1999. Military, public, and private laboratories were contacted to ascertain routine gonorrhea testing methods (culture versus nonculture). In 1998, 507 gonorrhea cases were reported to HDH. Of these, 256 (50%) were diagnosed by culture and underwent antimicrobial susceptibility testing at HSL. Antimicrobial susceptibility testing records of gonococcal isolates originating in Hawaii from HSL, GISP, and CDC were reviewed to identify CipR gonococcal isolates and determine their prevalence in Hawaii. From January 1990 through September 1999, 105 gonococcal isolates were identified that were CipR (n=48) or had intermediate resistance to ciprofloxacin (CipI)† (n=57). For CipR isolates, the median ciprofloxacin minimal inhibitory concentration (MIC) was 2.0 µg/mL (range: 1.0--16.0 µg/mL). The percentage of gonococcal isolates in Hawaii that were CipR increased from 1.4% (four of 290) in 1997 to 9.5% (22 of 231) in 1999 (Figure 1). Of the 105 patients with CipR/CipI gonorrhea, sex was known for 97; medical records were available for 81. The median age was 30 years (range: 16--53 years), and 68 (70%) were male. Of 79 with reported race/ethnicity, 42 (53%) were Asians/Pacific Islanders, and 20 (25%) were white. The median number of reported sexual partners during the preceding 30 days was one (range: 0--3). Five (9%) of 55 persons identified themselves as homosexual or bisexual. Nine (12%) of 73 reported antimicrobial use (fluoroquinolone use was reported by one patient) during the 30 days before diagnosis of gonorrhea. Thirty (48%) of 62 denied foreign travel during the 30 days before diagnosis or having a sex partner with a similar history; 72 (91%) of 79 were treated with ceftriaxone or cefixime for their gonorrhea. Of 75 CipR/CipI isolates, 48 (64%) were resistant to penicillin; 28 (37%) were penicillinase-producing N. gonorrhoeae. In addition, 33 (44%) were resistant to tetracycline; one had plasmid-mediated tetracycline resistance. Among isolates tested for susceptibility to other antimicrobial agents, no evidence was found of decreased susceptibility to ceftriaxone, cefixime, or azithromycin, or resistance to spectinomycin. N. gonorrhoeae with decreased susceptibility to azithromycin, Kansas City, MissouriDuring March--December 1999, GISP identified a cluster of 12 men with gonorrhea who had decreased susceptibility to azithromycin (AziDS)§. The patients were seen at the Kansas City, Missouri STD clinic. In February 2000, CDC, the Missouri Department of Health and the Kansas City Health Department investigated this cluster. Medical records of the 12 patients were reviewed. The median age was 33 years (range: 23--44 years), and 10 were black. Six reported sex with a commercial sex worker, and all 12 denied sexual contact with other men. Two were HIV infected. Two reported antimicrobial use during the 30 days before diagnosis. All 12 were treated with cefixime. The median MIC for azithromycin was 2.0 µg/mL (range: 1.0--4.0 µg/mL). Preliminary laboratory data, including antimicrobial susceptibility results, auxotype, serovar, and Lip subtype (4), suggest the gonococcal strains were identical among the 12 patients. All isolates were susceptible to ceftriaxone, cefixime, spectinomycin, ciprofloxacin, and penicillin. Eleven of the gonococcal isolates had intermediate resistance to tetracycline (MIC=1.0 µg/mL); the remaining isolate was resistant to tetracycline (MIC=2.0 µg/mL) but was within testing variability of the results for the other 11. Reported by: R Ohye, MS, V Lee, MS, P Whiticar, MA, P Effler, MD, Hawaii Dept of Health; H Domen, MS, Hawaii Dept of Health State Laboratory. G Hoff, PhD, J Joyce, R Archer, MD, Kansas City Health Dept, Kansas City; M Hayes, Missouri Dept of Health. J Hale, MS, K Holmes, MD, Seattle GISP Regional Laboratory, Univ of Washington, Seattle. L Doyle, MASCP, G Procop, MD, Cleveland GISP Regional Laboratory, Cleveland Clinic Foundation, Cleveland, Ohio. Epidemiology and Surveillance Br, Div of STD Prevention, National Center for HIV, STD and TB Prevention; Bacterial STD Br, Div of AIDS, STD and TB Laboratory Research, National Center for Infectious Diseases; and EIS officers, CDC. Editorial Note:Antimicrobial resistance is an ongoing challenge for gonorrhea treatment and control. These investigations highlight an increased prevalence of fluoroquinolone-resistant gonorrhea in Hawaii and the emergence in Kansas City of the first reported cluster of patients with AziDS gonorrhea. These reports are limited to describing data routinely documented in medical records. Interviews with the patients and prospective data collection at STD clinics in both areas will provide detailed information on risk factors (e.g., recent travel, recent antimicrobial use, and contact with commercial sex workers). CDC recommendations for gonorrhea therapy include use of either of two fluoroquinolone antimicrobials (ciprofloxacin or ofloxacin) because they are inexpensive, single-dose, oral medications (5). Fluoroquinolones are used widely in the United States to treat gonorrhea. Although infections with fluoroquinolone-resistant N. gonorrhoeae are endemic in many Asian countries (6), reports have documented only sporadic isolation of these strains in the United States (1). Excluding Hawaii, 0.2% of GISP isolates in 1999 were resistant to fluoroquinolones (1). Fluoroquinolone-resistant N. gonorrhoeae were first reported in the continental United States in 1995 in eight patients in Washington and one in Colorado (7). HDH and CDC recommend clinicians in Hawaii no longer use fluoroquinolone antimicrobials to treat gonorrhea. Absence of foreign travel among 48% of patients with CipR/CipI gonorrhea or their reported sex partners suggests CipR N. gonorrhoeae are being spread endemically in Hawaii. Therefore, for patients with gonorrhea in the United States, travel history, including sex partner travel history, should be obtained. If patients or their sex partners are likely to have acquired gonococcal infections in Hawaii, the Pacific Islands, or Asia, they should not be treated with fluoroquinolone antimicrobials; instead, ceftriaxone or cefixime should be used. For those unable to tolerate a cephalosporin, spectinomycin should be used. AziDS gonococcal isolates rarely have been reported in the United States or worldwide (8--10). Azithromycin is used widely to treat many community-acquired infections in the United States. In addition, a 1 g dose of azithromycin is recommended by CDC to treat Chlamydia trachomatis infections (5). However, this dose is inadequate to treat gonorrhea. Although a 2 g dose of azithromycin is approved for gonorrhea therapy by the U.S. Food and Drug Administration, CDC does not recommend routine treatment of gonorrhea infections with azithromycin because of cost and gastrointestinal intolerance at this dose (5). N. gonorrhoeae must be grown in culture for antimicrobial susceptibility testing to be performed. The increasingly widespread use of nonculture methods for gonorrhea diagnosis is a major challenge to monitoring antimicrobial resistance in N. gonorrhoeae. The changes in antimicrobial resistance patterns described in this report were identified only because culture was used as the diagnostic testing method in these sites and because susceptibilities were being measured through GISP for Kansas City. HSL is one of the few state public health laboratories performing antimicrobial susceptibility testing on all gonococcal isolates identified by culture. Clinicians who suspect or identify a N. gonorrhoeae infection treatment failure should submit a gonococcal culture specimen to the local health laboratory for susceptibility testing. CDC requests reports of treatment failures or resistant gonococcal isolates from clinicians or laboratories (National Center for HIV, STD and TB Prevention, Division of STD Prevention, telephone [404] 639-8373). CDC recommends that local health laboratories with the capacity to perform antimicrobial susceptibility testing on N. gonorrhoeae isolates routinely test for susceptibility to antimicrobials used locally for gonorrhea treatment (e.g., a fluoroquinolone, cefixime or ceftriaxone, azithromycin, and spectinomycin). Gonococcal isolates resistant to these classes of antimicrobials can be forwarded to CDC's Neisseria Reference Laboratory (telephone [404] 639-2134) for confirmation and further evaluation. References
* Resistance to ciprofloxacin is defined by the National Committee on Clinical Laboratory Standards as a minimal inhibitory concentration of >1.0 µg/mL by agar dilution or disk diffusion zone size of <27 mm. † Intermediate resistance to ciprofloxacin is defined by National Committee on Clinical Laboratory Standards as minimum inhibiting concentration=0.125--0.5 µg/mL by agar dilution or a disk diffusion zone size of 28--35 mm. § Decreased susceptibility to azithromycin was defined for this investigation as MIC of >1.0 µg/mL. No National Committee on Clinical Laboratory Standards criteria exist for decreased susceptibility or resistance to azithromycin for N. gonorrhoeae. Figure 1 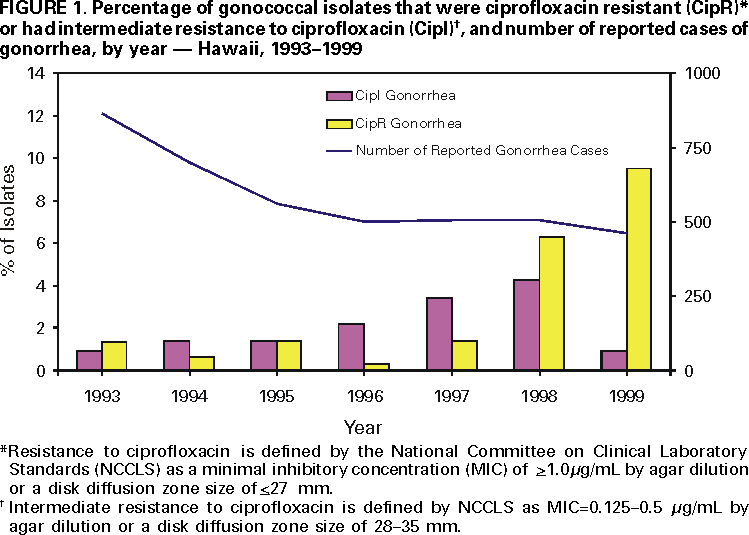 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/21/2000 |
|||||||||
This page last reviewed 5/2/01
|