 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Screening With the Prostate-Specific Antigen Test --- Texas, 1997Please note: An erratum has been published for this article. To view the erratum, please click here. Prostate cancer is the second leading cause of cancer-related deaths among men in Texas (1). From 1990 to 1997, the average annual number of prostate cancer-related deaths in Texas was 1900, and the average annual death rate was 20.9 per 100,000 population (1). An estimated 10,186 new prostate cancer cases will occur in 2000 in Texas (2). Several screening methods are available for early detection of prostate cancer, including digital rectal exam, transrectal ultrasound, and prostate-specific antigen (PSA) testing, which involves a simple phlebotomy (3). To assess the proportion of men in Texas receiving PSA testing and to identify factors associated with receipt of this testing, the Texas Department of Health added three questions to its 1997 Behavioral Risk Factor Surveillance System (BRFSS) survey relating to PSA testing. This report summarizes this analysis and indicates that approximately 37% of men aged >40 years had received PSA testing and that receipt of PSA testing was associated with a doctor's recommendation. BRFSS is a state-based, random-digit--dialed telephone survey of the civilian, noninstitutionalized U.S. population aged >18 years. In 1997, men aged >40 years who responded to the Texas BRFSS were asked, "Have you heard about the PSA blood test?", "Have you ever been told by a doctor that you should have a PSA blood test?", and "Have you ever had a PSA blood test?" Responses were weighted to provide statewide estimates; standard errors and 95% confidence intervals (CIs) were calculated, and univariate and multivatiate analyses were performed using SUDAAN. Among respondents, 60% (95% CI=55%--65%) said they had heard of the PSA test. Of those who had heard of the test, 52% (95% CI=45%--59%) were told by their doctor that they should receive the test. Of those who had heard of the test and whose doctor recommended it, 91% (95% CI=85%--96%) reported having received the test. Overall, 37% (95% CI=32%--42%) of men received the PSA test, representing approximately 62% of men who had heard of the test. Of those who were not told by a doctor to have the test, 24% (95% CI=14%--30%) received the test. Univariate analysis indicated that receiving the PSA test was associated with a doctor's recommendation (odds ratio [OR]=28.2; 95 CI=13.3--59.8) (Table 1). Other factors associated with receiving the test were having had a physical examination during the preceding 2 years (OR=5.4; 95 CI=2.6--11.0), being aged >50 years (OR=5.2; 95 CI=5.1--5.2), being covered by a health plan (OR=3.8; 95 CI=2.0--7.1), having ever received a proctoscopic examination (OR=3.4; 95 CI=2.0--5.7), being nonHispanic (OR=2.2; 95 CI=2.2--2.3), and having >16 years of education (OR=1.9; 95 CI=1.2--3.0). Logistic regression analysis indicated that receiving a PSA test was associated with a doctor's recommendation (adjusted OR=80.4; 95% CI=21.4--301.9), and being aged >50 years (adjusted OR=4.0; 95% CI=1.3--12.3). Reported by: K Condon, Behavioral Risk Factor Surveillance System Program, L Suarez, PhD, Office of the Associate Commissioner of Disease Control and Prevention, D Perrotta, PhD, State Epidemiologist, Texas Dept of Health. Epidemiology and Health Svcs Research Br, Div of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion; State Br, Div of Applied Public Health Training, Epidemiology Program Office; and an EIS Officer, CDC. Editorial Note:The findings in this report document a strong association between a doctor's recommendation and receipt of a PSA test, indicating that physician advice is a key determinant of whether men are tested. In Texas, approximately 60% of men who had heard of PSA testing reported receiving the test. Nearly all of the men whose doctor recommended the PSA test took that advice. Prostate cancer screening recommendations differ among national organizations. The American Cancer Society (ACS) recommends that men aged >50 years who receive an annual examination be offered the Digital Rectal Examination and the PSA test (4). ACS also recommends that men aged >40 years be informed about the risk for prostate cancer (5). In comparison, because no evidence exists that early detection and treatment influences the overall death rate from this disease and about half of the men who undergo surgical treatment of localized lesions experience side effects (e.g., incontinence and impotence) (6), the U.S. Preventive Services Task Force, the American College of Physicians, the American Society of Internal Medicine, the National Cancer Institute, the American Association of Family Practitioners, and the American College of Preventive Medicine do not advocate routine screening (7,8). Despite the conflicting recommendations, PSA testing has increased rapidly among asymptomatic men in the United States (9). The findings in this report are subject to at least four limitations. First, BRFSS questions did not distinguish prostate cancer screening from diagnostic testing. Some respondents may have received PSA testing as part of a diagnostic evaluation for symptoms or to monitor treatment for existing prostate cancer. Second, respondents may have had the PSA test performed, but did not know that it had been done. Third, among men for whom PSA testing was not recommended, it is not possible to distinguish men whose physicians discouraged screening from those whose physicians did not mention screening. Finally, because the survey did not ask men how they heard about the test, the proportion of men hearing about the test from sources other than their doctor is not known. The findings of this report suggest that interest in prostate cancer and awareness about the available screening tests for this disease is substantial. These data are consistent with information from other studies that indicate a substantial proportion of men aged >40 years have received PSA testing. Because PSA testing potentially can have an impact on statistics about prostate cancer incidence and outcomes, ongoing surveillance of the trends and determinants of the use of this procedure are warranted (8). As a result, CDC is incorporating questions about PSA testing in the 2001 BRFSS for every state. This effort, and continuing surveillance in states such as Texas, will provide information on the use of prostate cancer testing and facilitate a clearer delineation of the public health impact of this screening. Although prostate cancer screening recommendations vary, one consistent element is that physicians should counsel patients about the risks for and potential benefits of treatment for early prostate cancer so that patients can participate in the decision making about whether to be screened. To counsel patients effectively about the risks for and benefits of treatment of early prostate cancer, physicians need access to current information and to incorporate it into their practices. References
Table 1 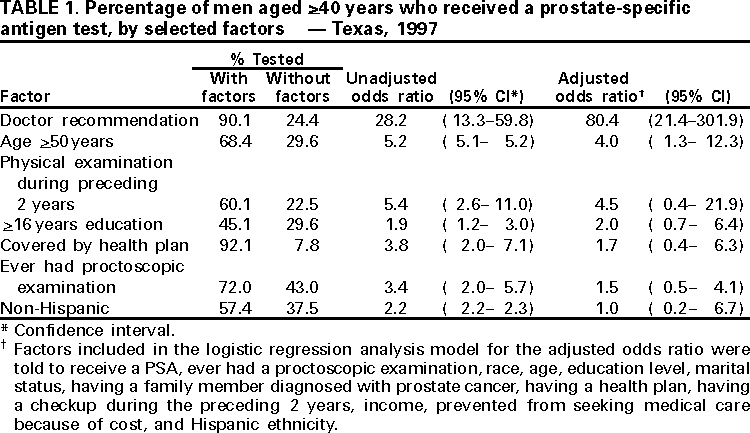 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/14/2000 |
|||||||||
This page last reviewed 5/2/01
|