 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Use of FDA-Approved Pharmacologic Treatments for Tobacco Dependence --- United States, 1984--1998Of the estimated 48 million adult smokers in the United States, approximately 16 million attempt to stop smoking cigarettes for at least 24 hours annually; another 2--3 million attempt to stop but cannot abstain for 24 hours (1). However, 1.2 million (2.5%) persons stop smoking each year (2). Although behavioral and pharmacologic methods increase abstinence rates (3), most cessation attempts are undertaken without the benefit of treatment (4). In 1984, the Food and Drug Administration (FDA) approved the first pharmacologic aid for smoking cessation, nicotine gum. Since then, other treatments have become available. This study estimates the number of quit attempts using FDA-approved pharmacologic aids during 1984--1998. The study results indicate that product use has changed over this period and that the availability of over-the-counter (OTC) products and the introduction of new products have increased pharmacologically assisted quit attempts. Information about the sales of prescription smoking-cessation products was obtained from the National Data Corporation* (NDC) by the Source Prescription Audit (SPA). By 1991, the pharmacy sample included approximately 25,000 pharmacies and 75 million prescriptions filled nationally each month; by 1998, sales data included 34,000 pharmacies and 150 million prescriptions covering approximately 66% of the total number of prescriptions in the United States. The total number of prescriptions was obtained through an agreement with PCS Health Systems, Inc., which provides electronic claims services for almost every retail pharmacy. Information about the sale of OTC products was based on data gathered by ACNielsen, a marketing research organization, that tallied purchases using an electronic Universal Product Code (UPC) scanner. Scanner data were collected from a sample of 10,000 outlets located primarily in the top 50 U.S. markets. Purchases from retail outlets without scanner technology were estimated from a sample of those stores. The combined sample was weighted to estimate total purchases from all outlets. Purchase data from a representative panel of 40,000 households were used to estimate the proportion of unit sales of OTC nicotine replacement therapy (NRT) products representing new uses or quit attempts. The panel of households used a UPC scanner placed in their home to scan purchases after shopping. A new use or quit attempt was counted when an OTC product appeared for the first time in a household's data during a particular calendar year. ACNielsen retail volume estimates were adjusted to project the total number of new OTC uses based on these data. In 1992, the availability of prescription nicotine patches increased the estimated number of pharmacologically assisted quit attempts per year from 1--2 million to approximately 7 million (Figure 1). The estimated number of quit attempts then decreased, ranging from 2 million to 3 million during 1993--1995, but increased to approximately 6 million in 1996, coinciding with the availability of nicotine gum and the nicotine patch as OTC products. The estimated number of pharmacologically assisted quit attempts increased in 1997 and remained at that level in 1998. By 1998, the nicotine patch accounted for 49% of the pharmacologically assisted quit attempts, nicotine gum, 28%; Zyban, 21%; and nicotine inhaler and nasal spray, <3%. To examine the relation of use to medication availability, data were aggregated into periods marked by the introduction of new treatments and changes in the regulatory status of treatments. In general, use has increased over time as availability improved, and to a lesser extent as new products were introduced. For example, the number of average monthly estimated quit attempts was 642,000 during May 1996--May 1997 when nicotine gum and patches became available OTC, compared with 259,000 during January 1993--April 1996. The introduction of Zyban increased average monthly estimated quit attempts to 708,000. Reported by: SL Burton, SmithKline Beecham Consumer Healthcare, Pittsburgh, Pennsylvania. JG Gitchell, Pinney Associates, Bethesda, Maryland.† S Shiffman, Smoking Research Group, Dept of Psychology, Univ of Pittsburgh, and Pinney Associates, Pittsburgh, Pennsylvania. Epidemiology Br, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Editorial Note:This report suggests that providing more pharmacologic options to smokers can increase the number of treatment-assisted quit attempts. The two largest increases in medication use occurred when prescription patches were introduced in 1992 and when nicotine gum and patches became available without a prescription. The initial increase in use that followed patch introduction was not maintained. A peak in consumer demand outstripped supply, making patches difficult to purchase. The shortage combined with possible smoker and physician disappointment in the patch's efficacy may have caused a decline during the next 3 years to one third of the 1992 volume. The increased sales coinciding with OTC marketing of patches and gum have been sustained for several years. This volume may be attributable to smokers' more realistic expectations about the role of NRT in quit efforts. New NRT and non-NRT products have been approved since 1996. The introduction of the non-nicotine prescription medication, Zyban, appears to have increased modestly the number of pharmacologically assisted cessation attempts. The introduction of two new prescription forms of NRT (i.e., nicotine nasal spray and oral inhaler) have had almost no impact on treatment use. This lack of effect may have occurred because of poor acceptance of these forms, poor promotion, or limits on the demand for and penetration of prescription NRT products when NRT is available without a prescription. Potential barriers to use of tobacco treatment medications include concerns about the safety and cost of the treatments. FDA has approved all of these treatments as safe and effective, and those approved for OTC availability were deemed sufficiently safe not to require physician screening or intervention. Treatment guidelines recommend that treatment of tobacco use be an insured medical benefit (3). A recent study in a health plan demonstrated that decreasing the costs of treatment increased use of treatment and the number of persons who quit smoking (5). This is important because the prevalence of smoking is higher among persons of low socioeconomic status; access to these treatments must be assured in these populations. The results of this study are subject to at least three limitations. First, estimates of use are based on sales data, prescription audits, and home scanning of purchases rather than direct questioning of users. It is not possible to determine whether a particular purchase represents a new quit attempt or the use of a product as a substitute for smoking in places where smoking is not allowed. In addition, the accuracy of pharmacy data may have improved over time as coverage increased, resulting in more accurate estimates for recent years. Second, prescription and OTC data are estimated by different methods and data sources. Prescription data may overestimate quit attempts because they may not adequately track successive prescriptions within a quit attempt. OTC data may underestimate quit attempts because they reflect only one quit attempt per household per year. Therefore, actual differences between prescription and OTC products may be greater than reported in these estimates. Finally, although shifts in use corresponded to major shifts in availability and marketing of medications, this study did not examine use in relation to concurrent events such as changes in smoking policies or legislation, higher cigarette prices, increased awareness of health issues, or shifts in population attitudes and beliefs. The health benefits of quitting are substantial and are realized within a few years of quitting (6). Promotion of quitting is vital to reduce death and disease caused by tobacco, and recommended levels of resources to be applied to treatment of tobacco dependence have been developed and disseminated by CDC (7). In addition, increased cessation rates will be essential to achieve the Healthy People 2010 objectives, (8) and the American Cancer Society's Challenge Goals for the Year 2015 (9). Public health authorities, including the World Health Organization, have called for an increased focus on the treatment of tobacco dependence to reduce tobacco-caused death and disease. Pharmacologic interventions double success rates (3); however, these interventions must be used for their effects to be observed. Data from this report suggest that increasing the number of treatment options and the availability of pharmacologic products increases use of these treatments. References
* Use of trade names and commercial sources is for identification only and does not imply endorsement by CDC or the U.S. Department of Health and Human Services. † Pinney Associates provides consulting services to SmithKline Beecham, which develops and markets smoking cessation medications. Figure 1 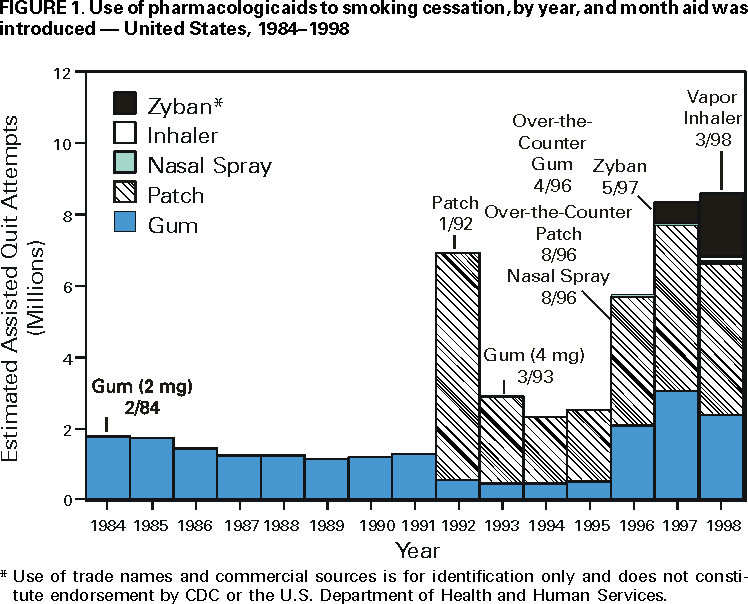 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/27/2000 |
|||||||||
This page last reviewed 5/2/01
|