 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Laboratory-Based Surveillance for Rotavirus -- United States, July 1996-June 1997Rotavirus infections are the most common cause of severe gastroenteritis among infants and young children worldwide (1,2). Each year in the United States, rotavirus infections account for an estimated 3.5 million cases of diarrhea, 500,000 physician visits, 50,000 hospitalizations, and 20 deaths among children aged less than 5 years (2). In addition, rotavirus accounts for 30%-50% of U.S. hospitalizations for diarrhea among children aged less than 5 years, including approximately 50% of hospitalizations for diarrhea during annual seasonal peaks, and is an important cause of nosocomial gastroenteritis (3). Rotavirus activity in the United States is monitored by the National Respiratory and Enteric Virus Surveillance System (NREVSS), a voluntary, laboratory-based system (4). This report summarizes surveillance from NREVSS during July 1996-June 1997. From July 1996 through June 1997, a total of 69 laboratories in 42 states participated in NREVSS and reported weekly to CDC the number of stool specimens tested for rotavirus by antigen-detection and electron microscopy methods and the number of positive results. Of 23,199 fecal specimens examined, 6183 (27%) were positive for rotavirus. Timing of rotavirus activity varied by geographic location; peak activity occurred first in the Southwest in November 1996 and last in the Northeast in April and May (Figure_1). * Data from Alaska and Hawaii were not available. Reported by: MT Bosley, Georgia Institute of Technology, Atlanta, Georgia. National Respiratory and Enteric Virus Surveillance System collaborating laboratories; Viral Gastroenteritis Section, Respiratory and Enteric Viruses Br, and Office of the Director, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: Seasonal increases in rotaviral diarrhea occur annually throughout the United States, and the temporal and geographic trends during the July 1996-June 1997 reporting period were similar to trends in previous years (4). The timing of rotavirus activity is sequential, beginning first in the Southwest in autumn and ending in the Northeast in mid- to late-spring; however, the time of peak activity in the Pacific Northwest is more variable than in other regions, occurring from winter to late spring. The reasons for the sequential pattern in rotavirus activity across the United States are unknown; it is not explained by a sequential introduction and diffusion of new rotavirus strains because rotavirus activity often is caused by a mixture of common strains that may vary between cities (5,6). NREVSS is the largest, nationally representative system for surveillance of rotavirus infections in the United States (4). This system uses an automated telephone reporting system to transmit reports from participating laboratories to CDC and allows timely analysis of rotavirus trends. Limitations of the system are that 1) some regions of the country are sparsely represented, and data are not reported from Alaska and Hawaii; 2) demographic or clinical data are not collected; and 3) specimens are not routinely submitted for confirmation or strain characterization. The large disease burden and high cost associated with rotavirus infections in the United States have been the impetus for development of rotavirus vaccines. Two human-animal reassortant vaccines have been found to be safe and effective (7-10), and a vaccine is under review for licensure for use among U.S. children; both the Advisory Committee of Immunization Practices and the Committee on Infectious Diseases of the American Academy of Pediatrics are considering recommendations for the use of the vaccine in children. The prospect of a program for childhood vaccination against rotavirus in the United States highlights the need for continued surveillance for this infection. Laboratory-based surveillance has helped characterize the spatiotemporal trends of rotavirus infections and provides a baseline for monitoring changes in the epidemiology of these infections following vaccine introduction. Efforts to enhance rotavirus surveillance should include surveillance for rotavirus-associated diarrheal outcomes, particularly hospitalizations, and for rotavirus strains. These measures also will assist in assessing vaccine program effectiveness and the potential emergence of novel or unusual rotaviruses. References
* A short animation showing rotavirus activity by week is available on the World-Wide Web at ftp://ftp.cdc.gov/pub/Publications/mmwr/wk/rota9697.gif. Figure_1 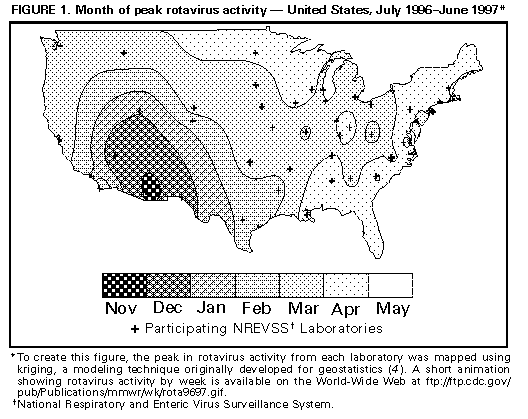 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|