 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Creutzfeldt-Jakob Disease Associated with Cadaveric Dura Mater Grafts -- Japan, January 1979-May 1996In 1997, a nongovernmental surveillance group for Creutzfeldt-Jakob disease (CJD) in Japan reported to the Ministry of Health and Welfare its analysis of a 1996 mail questionnaire survey of neurologic, psychiatric, and neuropathologic institutions throughout Japan. This analysis identified 829 patients with CJD diagnosed by physicians during January 1979-May 1996, including a large number (43 patients) who had received a cadaveric dura mater graft during a neurosurgical (42) or orthopedic (one) procedure during 1979-1991. This report presents a summary of features of these 43 cases, which indicated that at least 41 of these patients had received dura mater grafts from the same processor, and describes CJD in the most recent recipient of a dura mater graft. The findings indicate that an international outbreak of CJD associated with a single brand of dura mater grafts is larger than previously recognized and that recipients of contaminated grafts may remain at risk for CJD at least 16 years following receipt of grafts. Summary Findings Of the 4017 institutions surveyed, 2962 (74%) responded. Follow-up investigation of the 43 CJD cases associated with dura mater grafts revealed that at least 41 (95%) persons had received a single brand of dura mater graft, LYODURA{Registered} *, processed by B. Braun Melsungen AG. The grafts had been processed before May 1987, when the company revised its procedures for collection and processing dura. The revised procedures, designed to reduce the risk for CJD transmission, included conversion from batch to individual processing of dura mater and treatment of each dura mater graft with 1.0 normal sodium hydroxide (NaOH). The 43 patients with CJD had onsets of illness from September 1985 to May 1996 (Figure_1). The mean (plus or minus 1 standard deviation) age of the 43 patients at onset of dura mater graft-associated CJD was 53 years (plus or minus 13 years) compared with a mean age at onset (63 years {plus or minus 10 years}) of the other CJD cases identified in this survey (pless than 0.05); of the 43 patients who had received a dura mater graft, eight were aged less than 40 years at onset of CJD. The mean latency period between receipt of a dura mater graft and onset of CJD was 89 months (plus or minus 44 months) (range: 16-193 months). Neuropathologic examinations were performed for 10 decedents who underwent autopsy; typical spongiform degeneration was present in the cerebral and cerebellar cortex of all 10. Of the 43 CJD patients, 42 received their dura mater graft during 1979-1989 (Figure_2). All but one of the 42 patients were reported to have received LYODURA{Registered} that had been processed without exposure to NaOH. The brand of graft used in one patient who had undergone a neurosurgical operation in 1985 was unknown; however, LYODURA{Registered} was the only brand of dura mater graft used at the hospital. A total of 33 (77%) of the patients received their grafts during 1983-1987 (Figure_2), when an estimated 100,000 patients may have received LYODURA{Registered} in Japan. All of these 33 patients died of CJD within 12 years after receipt of the grafts (approximately 1 case of CJD per 3000 LYODURA{Registered} recipients). None of the lot numbers of the dura mater grafts used in the 43 patients could be identified. Case Description The most recent recipient of a dura mater graft among the 43 graft-associated CJD patients was a woman aged 65 years at the time of onset of CJD. She had two neurosurgical procedures in 1991 to repair a cerebral arterial aneurysm (one in September and one in October); dura mater grafts were used during both operations. In February 1994, 28 months after the second operation, she developed progressive dysphagia, dysarthria, and unsteady gait, followed within a few weeks by dementia. In April 1995, she developed generalized myoclonic jerks and akinetic mutism. An electroencephalograph showed a 6- to 10-Hz background rhythm with the periodic synchronized slow activity complexes typical of CJD. Examination of the cerebrospinal fluid revealed a normal protein level and cell count. A magnetic resonance imaging scan showed marked cerebral and cerebellar atrophy. The patient died in October 1995, and no autopsy was performed. Neither the brand nor year of processing of the dura mater grafts used in this patient in 1991 could be determined. However, the hospital in which both neurosurgical procedures were performed opened in 1989 and reported using only two brands of dura mater grafts in 1991, LYODURA{Registered} and Tutoplast (Pfrimmer-Viggo GmbH+Co, Erlangen, Germany). The investigation suggested that in this patient, the use of LYODURA{Registered} processed before May 1987 was unlikely but could not be ruled out. Reported by: T Sato, MD, Chairman, Japanese National CJD Surveillance Group; K Hoshi, MD, H Yoshino, MD, J Urata, MD, Kohnodai Hospital, National Center for Neurology and Psychiatry, Ichikawa-shi, Chiba-ken, Japan. Y Nakamura, MD, H Yanagawa, MD, Dept of Public Health, Jichi Medical Univ, Tochigi-ken, Japan. Office of the Director, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: In June 1987, after an investigation of the first reported LYODURA{Registered}-associated CJD case (1), CDC published a description of differences between the processing of LYODURA{Registered} and other similar products and suggested that LYODURA{Registered} may be associated with a higher risk for transmitting CJD than other dura mater products used in the United States (2). Also in June 1987, representatives of B. Braun Melsungen AG reported that as of May 1, 1987, their procedures for collecting and processing dura were revised to reduce the risk for CJD transmission (3). By including the present report from Japan, the total number of reported LYODURA{Registered}-associated CJD cases has now increased to at least 61 worldwide (3-5). Despite limitations in the information about the number and subsequent vital status of recipients of LYODURA{Registered} and other dura mater grafts, the findings and evidence in this report strongly indicate that the LYODURA{Registered} grafts were the vehicle of transmission of the CJD agent in Japan. This evidence includes the high overall estimated incidence of CJD among the LYODURA{Registered} graft recipients and the identification, almost exclusively, of the same brand of graft produced during the same early period that had been associated with at least 21 other reported CJD cases worldwide. In comparison with the strong evidence of CJD transmission by LYODURA{Registered} produced before May 1987, the evidence in Japan is substantially weaker for CJD transmission by a non-LYODURA{Registered} brand of graft or a LYODURA{Registered} graft produced after May 1987; such an association was reported as likely in only one CJD patient who was not unusually young. However, for this patient, the investigation cannot exclude a causal relation between disease and receipt of a graft other than LYODURA{Registered} produced before May 1987. Even stringent donor screening and processing of each dura separately to avoid possible cross-contamination may not completely eliminate the potential for an infectious graft. In addition, the treatment of dura with NaOH may not inactivate all of the infectious agent that may be present (6). Therefore, surgeons should be aware of the possibly inherent risk for CJD transmission by dura mater grafts and may want to consider the alternative use of autologous fascia lata, fascia temporalis, or synthetic substitutes (4). The cases described in this report indicate that recipients of contaminated grafts may remain at risk for CJD at least 16 years after receiving grafts. In the United States, patients who have rapidly progressive dementing illnesses consistent with CJD and who have received an allograft should be reported through their respective local or state health departments to CDC's Division of Viral and Rickettsial Diseases, National Center for Infectious Diseases, telephone (404) 639-3091.
References
* Use of trade names and commercial sources is for identification only and does not imply endorsement by CDC or the U.S. Department of Health and Human Services. Figure_1 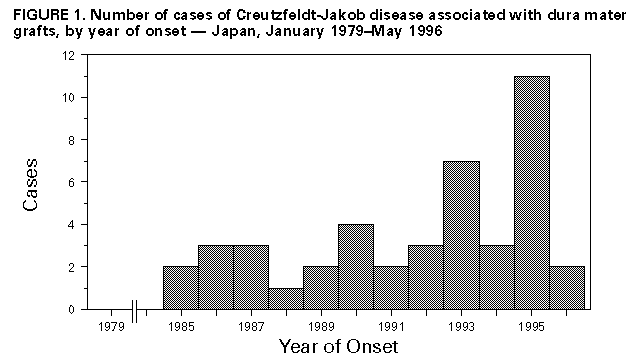 Return to top. Figure_2 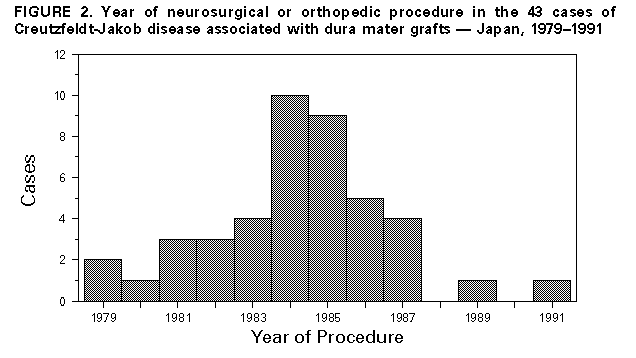 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|