 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Outbreak of Invasive Group A Streptococcus Associated with Varicella in a Childcare Center -- Boston, Massachusetts, 1997Group A Streptococcus (GAS) causes common childhood diseases such as streptococcal pharyngitis and impetigo and can cause severe, life-threatening invasive disease including streptococcal toxic-shock syndrome and necrotizing fasciitis. Invasive GAS disease occurs when GAS infects a normally sterile site. Clusters of invasive GAS infection previously had not been reported among children in school or childcare centers (CCCs). However, on February 13, 1997, the Boston Public Health Commission received reports of cases of concurrent invasive GAS and varicella infection among two of 14 children in the same CCC classroom. Because of the potential for further spread of invasive disease, the Boston Public Health Commission initiated an investigation of these cases. This report describes the findings of the investigation, including risk factors for infection, and recommended prevention measures. The findings indicate the potential for widespread GAS infection and carriage in CCCs and suggest that, in this outbreak, antecedent use of varicella vaccine would have prevented cases of invasive GAS. Case Descriptions On February 2, a previously healthy 4-year-old girl (patient who had had onset of varicella on January 30 was taken to a local hospital because of swelling, tenderness, warmth, and redness over her left upper arm and shoulder. Purulent skin lesions were not present, and a blood culture was negative. The patient was admitted to the hospital with a diagnosis of cellulitis and received intravenous clindamycin, but her symptoms did not improve. She underwent surgical exploration for possible necrotizing fasciitis and subsequently received a total fasciotomy of her left arm. Cultures of tissue specimens obtained at surgery grew GAS, serotype M1T1. On February 6, submental abscess was diagnosed in a 3-year-old classmate of patient 1 (patient 2), 7 days after onset of varicella infection. No obviously infected lesions were located over or near the abscess, and a blood culture was negative. The abscess was incised and drained, and contents grew GAS, serotype M1T1. Investigation of the CCC In February 1997, a total of 39 children aged 1-4 years were enrolled in the CCC. The children were divided into three classrooms by age group, and groups were separated throughout the day except for 2 hours of outdoor play. To assess the prevalence of GAS carriage associated with the CCC, during February 17-19 throat swab specimens were obtained from all children attending the CCC, their household contacts, and all CCC employees. Cases of GAS infection were identified by review of hospital records and telephone interviews with physicians. Four case definitions were used to categorize GAS status: a case of invasive GAS infection was defined as isolation of GAS from a normally sterile site; a case of noninvasive GAS infection was defined as identification of GAS from the throat of an ill person (strep throat) either by rapid-antigen test or culture; a case of possible GAS infection was defined as identification of a clinical illness commonly caused by GAS; and a case of GAS carriage was defined as isolation of GAS from the throat culture of a well person. Of the 14 classmates of patients 1 and 2, three had strep throat, and two had GAS carriage. Two of three available isolates (one obtained from a child with strep throat and one from a carrier of GAS) were serotype M1T1. In addition, two cases of possible GAS infection were identified: one with an infected varicella lesion and the other with leg cellulitis; however, cultures were not obtained from the lesions (Figure_1). Of the 25 children in other classrooms, one had scarlet fever, serotype M1T1, and one was a carrier of GAS of another serotype. Of the 92 household contacts, three had strep throat, and their isolates were not available for serotyping. GAS carriage was present in two persons (one was serotype M1T1 and the other was a different serotype). Of the 13 CCC workers, GAS carriage, not M1T1, was present in one person. The child with scarlet fever and the household contact carrier of serotype M1T1 were both siblings of classmates of patients 1 and 2. Identification of Risk Factors for Infection Risk factors for GAS infection among classmates of patients 1 and 2 were identified from responses by parents on self-administered written questionnaires. All nine cases of test-confirmed (by culture or rapid-antigen test) and possible GAS infection occurred in persons with varicella infection. Of the 14 classmates of patients 1 and 2, a total of 12 were susceptible to varicella at the start of the school year: one had a previous history of varicella, and one had been vaccinated against varicella. The first case of varicella occurred on January 15; of the other 11 susceptible children, 10 had onsets of varicella during January 29-February 1 (Figure_2). Of these, seven were identified with GAS infection or carriage, and two had onset of possible GAS disease 3-14 days following onset of varicella. Of the 11 children who were not receiving antibiotics and whose GAS status was test-confirmed, seven who spent greater than 30 hours per week at the CCC had documented GAS infection or carriage compared with none of the four children who spent less than or equal to 30 hours (Fisher's exact test, p less than 0.01). A total of 112 environmental surfaces were cultured to assess the possible role of fomites in disease transmission. These surfaces included handles and other sites that a child was likely to grip (70) and any toy (e.g., toy food and phones) that a child was likely to place in his or her mouth (42). Of the 112 samples, six pieces of flat plastic food were positive for GAS; five were serotype M1T1. Varicella vaccine was recommended and provided free for all children, CCC staff, and household contacts considered to be susceptible. In addition, to prevent additional cases of GAS infection, prophylactic antibiotic therapy was recommended for all carriers of GAS and all classmates of patients 1 and 2 regardless of culture results. The specific antibiotic therapy was prescribed by the patient's physician. Reported by: MA Barry, MD, K Matthews, P Tormey, Boston Public Health Commission; BT Matyas, MD, SM Lett, MD, JA Ida, MS, DM Hamlin, MS, P Kludt, MPH, K Yih, PhD, A DeMaria, Jr, MD, State Epidemiologist, Massachusetts Dept of Public Health. Epidemiology and Laboratory Section, Respiratory Diseases Br, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases; Child Vaccine Preventable Disease Br, Epidemiology and Surveillance Div, National Immunization Program, CDC. Editorial NoteEditorial Note: Invasive GAS infection is a known complication of varicella infection in children. Previous reports indicate that among children with GAS bacteremia, 6%-17% of cases were associated with antecedent varicella infection (1-3). In Toronto, an analysis of active surveillance data for invasive GAS infection suggested a substantially increased risk for infection in children during the 2-week period following onset of varicella infection (2). Other reports have documented community clusters of varicella complicated by invasive GAS infection (4,5). For example, in 1994, a total of 24 previously healthy children (median age: 3 years; range: 6 months-8 years) in southern California developed invasive GAS infection after varicella infection (4). The Advisory Committee on Immunization Practices and American Academy of Pediatrics recommend routine varicella vaccination for infants aged 12-18 months and vaccination of susceptible children, adolescents, and adults (6,7). Because of the increased risk for invasive GAS infection in persons with antecedent varicella and the occurrence of community clusters of invasive GAS in persons with varicella, the use of varicella vaccine is one strategy for preventing some cases of invasive GAS infection. Because information about the epidemiology of GAS in CCCs is limited, there are no published recommendations about prevention strategies following identification of one or more cases of invasive GAS infection in CCCs. In Alabama, a fatal case of invasive GAS infection in a child attending a CCC led to identification of GAS carriage in 25% of all children in six of nine classrooms attending the CCC (8). In Sweden, after a child and teacher in a CCC had onset of GAS pharyngitis, GAS infection or carriage occurred in 61% of the children in the CCC's two classrooms within 16 days of identification of the index case (9). In the Boston outbreak, few children were infected outside the classroom of patients 1 and 2, possibly reflecting the greater separation between the groups, the ability of 4-year-old children to control secretions, and/or the role of fomites in transmission. Results of this investigation suggest that age and CCC characteristics may be important factors in developing prevention strategies. The role of the plastic food in transmitting GAS in this outbreak is unclear; although 4-year-old children typically do not place toys in their mouths, toy "food" may have encouraged such behavior. Guidelines for sanitation in CCCs state that "toys that are placed in children's mouths...should be set aside to be cleaned with water and detergent, disinfected, and rinsed before handling by another child" (10). Individual CCCs should determine whether they should have toy "food" based on their ability to enforce this standard. References
Figure_1 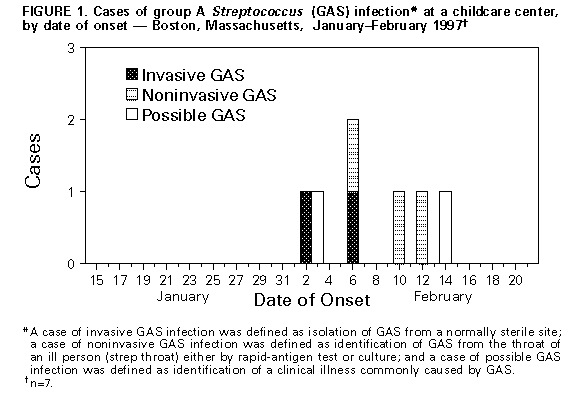 Return to top. Figure_2 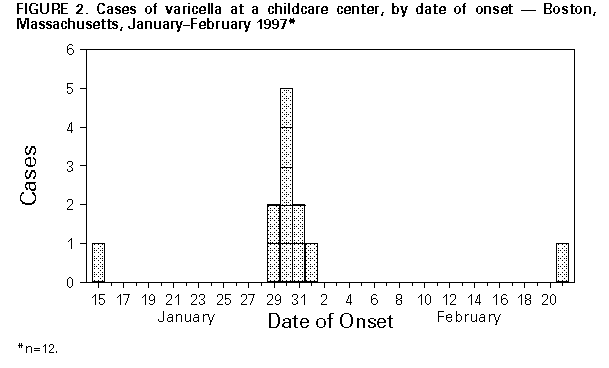 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|