 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevention of Varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP)Summary These recommendations represent the first statement by the Advisory Committee on Immunization Practices (ACIP) on the use of live, attenuated varicella virus vaccine -- VARIVAX -- manufactured by Merck and Company, Inc. and licensed in March 1995 for use in healthy persons greater than or equal to 12 months of age. In addition to presenting information regarding vaccine, this statement updates previous recommendations concerning the use of varicella zoster immune globulin (VZIG) as prophylaxis against varicella (MMWR 1984;33:84-90,95-100). INTRODUCTION Varicella (i.e., chickenpox) is a highly contagious disease caused by varicella zoster virus (VZV). Secondary attack rates for this virus may reach 90% for susceptible household contacts. VZV causes a systemic infection that usually results in lifetime immunity. In otherwise healthy persons, clinical illness after reexposure is rare; such illness is more likely to occur among immunocompromised persons. However, as with other viral diseases, reexposure to wild-type varicella often leads to reinfection that boosts antibody titers without causing clinical illness or detectable viremia. VZV remains dormant in sensory-nerve ganglia and may be reactivated at a later time causing herpes zoster (i.e., shingles) -- a painful vesicular rash usually appearing in a dermatomal distribution of one or two sensory-nerve roots. Among children, varicella is usually a self-limited disease that lasts 4-5 days and is characterized by fever, malaise, and a generalized vesicular rash typically consisting of 250-500 lesions. Adolescents, adults, and immunocompromised persons usually have more severe disease and are at higher risk for complications. Primary subclinical infection with VZV is rare for persons of all ages. EPIDEMIOLOGY OF VARICELLA General VZV is transmitted from person to person by a) direct contact, droplet, or aerosol from vesicular fluid of skin lesions or b) secretions from the respiratory tract. The virus enters the host through the upper-respiratory tract. The average incubation period for varicella is 14-16 days; however, this period can range from 10 to 21 days. The period of contagiousness of infected persons is estimated to begin 1-2 days before the onset of rash and end when all lesions are crusted, which is usually 4-5 days after onset of rash. Persons who have progressive varicella may be contagious longer, presumably because their immune response is depressed, which allows viral replication to persist. Because varicella develops in nearly all persons who live in the United States, the incidence is estimated to approximate the birth cohort. Data from the National Health Interview Survey (NHIS) for the period 1980-1990 indicated that an estimated 3.7 million cases occur annually (annual incidence rate: 1,498 cases per 100,000 population) (CDC, unpublished data). Varicella is not a nationally notifiable disease, and surveillance data are limited. In 1994, only 28 states, the District of Columbia, and New York City reported cases to CDC's National Notifiable Disease Surveillance System (NNDSS), and reporting within states was incomplete. Reporting efficiency is only an estimated 4%-5%. Age-specific incidence data were derived from NHIS for the period 1980-1990 (CDC, unpublished data). An estimated 33% of cases occurred in preschool-age children (i.e., children 1-4 years of age {annual incidence rate: 82.8 cases per 1,000 children}), and 44% occurred in school-age children (i.e., children 5-9 years of age {annual incidence rate: 91.1 cases per 1,000 children}) (Figure_1). More than 90% of cases occurred in persons less than 15 years of age, and few cases occurred in persons greater than 49 years of age. Epidemiologic and serologic studies confirm that greater than 90% of adults are immune to VZV (CDC, unpublished data;1). Rates of immunity may be lower for adults who were raised in certain tropical or subtropical areas (e.g., Puerto Rico) (2). Otherwise healthy children and adolescents (i.e., persons less than 15 years of age) comprise the largest proportion (80%) of an estimated 9,300 annual varicella-related hospitalizations. However, the rate of complications is substantially higher for persons greater than or equal to 15 years of age and for infants (i.e., children less than 1 year of age). The most common complications of varicella, which can result in hospitalization, are bacterial infections of skin lesions, pneumonia, dehydration, encephalitis, and hepatitis. Since the association between Reye syndrome and aspirin use was identified, Reye syndrome, which was once considered a common complication resulting from varicella infection, now rarely occurs (3,4). The mean annual number of persons who died in the United States as a result of complications of varicella decreased from 106 persons during 1973-1979 to 57 persons during 1982-1986. This decrease may have resulted from a) the substantial reduction in cases of Reye syndrome, b) the availability of acyclovir, c) the selective use of varicella zoster immune globulin (VZIG), and d) improvements in supportive care. However, during 1987-1992, the mean annual number of varicella-related deaths increased to 94 persons; the cause of this increase is unknown. The case-fatality rate is lower for children and adolescents 1-14 years of age than for infants (0.75 cases per 100,000 children and 6.23 cases per 100,000 infants). Among persons greater than or equal to 15 years of age, the risk for death increases with age, from 2.7 per 100,000 among persons 15-19 years of age to 25.2 per 100,000 among persons 30-49 years of age (CDC, unpublished data). Although the varicella-related mortality rate among children generally is low, during periods of increased varicella incidence, the circulation of virulent strains of group A streptococci (which are more likely to cause invasive, fatal infections) can result in an unusually high number of hospitalizations and deaths among children (5,6). Prenatal and Perinatal Exposure Although prenatal infection is uncommon because most women of childbearing age are immune to VZV (7), varicella in pregnant women is associated with a risk for VZV transmission to the fetus or newborn. Intrauterine VZV infection may result in congenital varicella syndrome, clinical varicella (during the newborn period), or clinical zoster (during infancy or early childhood) (8-17). Infants who are prenatally exposed to VZV, even if asymptomatic, may have measurable varicella-specific IgM antibody during the newborn period, have persistent varicella-specific IgG immunity after 1 year of age without a history of postnatal varicella, or demonstrate positive lymphocyte transformation in response to VZV antigen (8). Congenital varicella syndrome, first recognized in 1947 (11), can occur among infants born to mothers infected during the first half of pregnancy and may be manifested by low birthweight, cutaneous scarring, limb hypoplasia, microcephaly, cortical atrophy, chorioretinitis, cataracts, and other anomalies. Aggregate results from prospective studies (8,13-15) indicate that congenital varicella syndrome developed in four (0.7%) of 564 infants born to mothers who had varicella during the first trimester of pregnancy. In a prospective study conducted in the United Kingdom and West Germany from 1980 to 1993, a higher risk for congenital varicella syndrome was observed when maternal infection occurred during 13-20 weeks' gestation than when infection occurred from conception through 12 weeks' gestation (2% versus 0.4%, respectively) (14). In this same population, herpes zoster developed during infancy or early childhood in four (0.8%) of 477 infants who were exposed to VZV during 13-24 weeks' gestation and six (1.7%) of 345 infants who were exposed during 25-36 weeks' gestation. The onset of varicella in pregnant women from 5 days before to 2 days after delivery results in severe varicella infection in an estimated 17%-30% of their newborn infants. These infants are exposed to VZV without sufficient maternal antibody to lessen the severity of disease. The risk for death among neonates has been estimated to be 31% among those infants whose mothers had onset of rash 0-4 days before giving birth (16). This estimated risk was based on a limited number of infant deaths and may be inflated because some cases resulted from selective reporting and occurred before neonatal intensive care was available. When cases were reevaluated, several infants had been at higher risk for death because of low birthweight. In at least one case, another cause of death had been probable (17). Although the risk for death among neonates who do not receive VZIG intervention is likely substantially lower than was previously estimated, VZIG has had a salutary effect on neonatal disease. For example, although only 21 neonatal deaths were reported in the United Kingdom during a 20-year period before VZIG was available, the proportion of deaths among neonates infected with varicella decreased from 7% to none after routine use of VZIG (18). Nosocomial Transmission Nosocomial transmission of VZV is well recognized (19-29), and guidelines for the prevention of nosocomial VZV infection previously have been published (30). Guidelines concerning infection control for hospital personnel are being developed (CDC, unpublished data). Sources of nosocomial exposure have included patients, hospital staff, and visitors (e.g., the children of hospital employees) who are infected with varicella or herpes zoster. In hospitals, airborne transmission of VZV has been demonstrated when varicella has occurred in susceptible persons who have had no direct contact with the index case-patient (31-35). Although severe varicella disease and complications can occur in all susceptible, hospitalized patients, certain conditions are associated with higher risk. Patients at high risk for severe disease and complications include a) premature infants born to susceptible mothers, b) infants who are born at less than 28 weeks' gestation or who weigh less than or equal to 1,000 g (regardless of maternal immune status), and c) immunocompromised persons of all ages -- including persons who are undergoing immunosuppressive therapy, have malignant disease, and are immunodeficient. Strategies for managing clusters of VZV infection in hospitals have included isolating patients who have varicella and susceptible patients who have been exposed to the virus; controlling air flow; using rapid serologic testing; furloughing or screening exposed, susceptible personnel daily for skin lesions, fever, and systemic symptoms; and temporarily reassigning susceptible personnel to locations remote from patient-care areas (36-43). Appropriate isolation of hospitalized patients who have confirmed or suspected VZV infection can reduce the risk for transmission to personnel. Only personnel who are immune to varicella should care for these patients. If susceptible personnel are exposed to varicella, they are potentially infective 10-21 days after exposure and are often furloughed, usually at substantial cost. The use of VZIG following exposure can be costly, does not necessarily prevent varicella, and may prolong the incubation period by greater than or equal to 1 week, thus extending the time during which personnel should not work in patient areas. Herpes Zoster Following varicella, VZV persists in a latent form in sensory-nerve ganglia without any clinical manifestation. The latent virus can be reactivated, causing herpes zoster. Approximately 15% of the population will experience herpes zoster during their lifetimes (44). Herpes zoster develops most frequently among immunocompromised persons and the elderly. Disseminated herpes zoster with generalized skin eruptions and central nervous system, pulmonary, hepatic, and pancreatic involvement is more likely to occur in immunocompromised persons than in the general population. VZV can be transmitted from the lesions of patients who have herpes zoster to susceptible contacts, although the likelihood of transmission of VZV from herpes zoster is much less than that from primary varicella. Transmission of VZV from patients who have herpes zoster results in primary varicella in susceptible contacts. VARICELLA ANTIBODY TESTING A reliable history of varicella is a valid measure of immunity. Because the rash is distinctive and subclinical cases rarely occur, most parents know if their child has had varicella. A negative history of varicella substantiated by a parent may be more accurate than a self-reported negative history given by an adult. Data from one study indicated that the attack rate following household exposure in parents who reported themselves as being susceptible was 5%; however, among children whose parents reported them as being susceptible, the attack rate was 87%. In children with positive histories, the attack rate was 7% (45). Serologic tests have been used to assess the accuracy of reported histories of varicella (1,26,42,46,47). In adults, a positive history of varicella is highly predictive of serologic immunity (97%-99% of persons are seropositive); however, the majority of adults who have negative or uncertain histories are also seropositive (71%-93%). The appropriateness of a laboratory test to detect antibody to VZV depends on the purpose for obtaining the information; tests differ in their ability to detect antibody acquired from natural varicella versus antibody acquired from vaccination -- levels of which are lower than those following natural infection. Likewise, tests that rapidly assess the susceptibility of persons at high risk who are exposed to varicella differ from those used in serologic surveys. Certain tests require equipment or techniques that are not appropriate for general diagnostic laboratories. Thus, the criteria for selection of an antibody-detection assay include test sensitivity and specificity, the length of time required to obtain results, and availability of the assay. Many methods have been used to detect antibody to VZV, including complement fixation (CF), indirect fluorescent antibody (IFA), fluorescent antibody to membrane antigen (FAMA), neutralization (N), indirect hemagglutination (IHA), immune adherence hemagglutination (IAHA), radioimmunoassay (RIA), latex agglutination (LA), and enzyme-linked immunosorbent assay (ELISA) (48-60). IFA, FAMA, N, and RIA are sensitive tests, but they are time consuming and have requirements that make them unsuitable for use in general diagnostic laboratories. The CF test has been widely used but is the least sensitive test; antibody may diminish to levels undetectable by CF several months after natural varicella infection. RIA and ELISA are equal in sensitivity; both tests are tenfold more sensitive than IFA and twentyfold more sensitive than CF (54). ELISA tests, which are commercially available, range in sensitivity from 86% to 97% and range in specificity from 82% to 99% in detecting antibody after natural varicella infection. A highly sensitive gpELISA using purified viral glycoproteins as antigens has been used in clinical trials for the large-scale testing of immunogenicity of varicella virus vaccine, but it is not commercially available (58). A commercially available LA test using latex particles coated with VZV glycoprotein antigens can be completed in 15 minutes and does not require special equipment (59). The sensitivity and specificity of the LA test are comparable to those of FAMA in detecting antibody response following natural varicella infection, but the LA test is less sensitive in detecting antibody following vaccination; for both purposes, the LA test generally is more sensitive than commercial ELISAs. The LA test has detected antibody for up to 11 years after varicella vaccination (60). ACYCLOVIR FOR THE TREATMENT AND PREVENTION OF VARICELLA Acyclovir is a synthetic nucleoside analog that inhibits replication of human herpes viruses, including VZV. Intravenous acyclovir has been available since the early 1980s for use in immunocompromised persons who have varicella and, when administered within 24 hours of onset of rash, has been effective in reducing the morbidity and mortality associated with varicella (61-63). In 1992, the Food and Drug Administration (FDA) approved the use of oral acyclovir for the treatment of varicella in otherwise healthy children. Approval was based on placebo-controlled, double-blind studies (64-65) that indicated that beneficial clinical effects occurred (i.e., a decrease in the number of days in which new lesions appeared, the duration of fever, and the severity of cutaneous and systemic signs and symptoms) when acyclovir was administered within 24 hours of onset of rash. No serious adverse events occurred during the period of drug administration. Acyclovir did not decrease transmission of varicella or reduce the duration of absence from school. The rate of complications from varicella is low for healthy children. Data from these studies could not determine whether acyclovir had a significant effect on this rate because too few complications occurred. In these studies, antibody titers following infection in children receiving acyclovir were not substantially different from titers of children in the control group (64,65). Clinical trials among adolescents and adults (66-68) have indicated that acyclovir is safe and effective in reducing the duration and severity of clinical illness if the drug is administered within 24 hours of the onset of rash. The Committee on Infectious Disease of the American Academy of Pediatrics (AAP) published a statement on the use of acyclovir (69). The Committee did not consider the administration of acyclovir to healthy children to be beneficial enough to justify routine administration to such children; however, the Committee acknowledged that certain circumstances may justify the minimal clinical benefit. Complications and more severe varicella may occur in adolescents and adults or in secondary case-patients who live in the households of infected children; therefore, AAP considered such situations appropriate for the use of this drug. The safety of systemic acyclovir therapy among pregnant women has not been established. Although studies involving animals have not indicated teratogenic effects, adequate, well-controlled studies among pregnant women have not been conducted. Acyclovir is classified as Category C in the FDA use-in-pregnancy rating (i.e., risk cannot be ruled out, but potential benefits may justify the possible risk) (Burroughs Wellcome Company, Zovirax package insert). AAP does not recommend oral acyclovir for pregnant women; however, in instances of serious, viral-mediated complications (e.g., pneumonia), AAP stated that intravenous acyclovir should be considered. Burroughs Wellcome Company maintains the Acyclovir in Pregnancy Registry to monitor the maternal-fetal outcomes of pregnant women who are administered systemic acyclovir. The registry is a collaborative effort of Burroughs Wellcome Company, CDC, and academic epidemiologists. * Two nucleoside analogs, acyclovir and famciclovir, have been approved by FDA for treating herpes zoster. If administered within 72 hours of rash onset, acyclovir has accelerated the rate of cutaneous healing and has reduced the severity of acute necrosis in adults who have herpes zoster (70). Oral famciclovir, when administered during the same period of time, has similar efficacy (71). The prophylactic use of acyclovir in susceptible children following household exposure to varicella recently has been studied. These studies indicated fewer and less severe cases among children administered acyclovir than among children in the control group. Data from one study indicated that varicella developed in 16% of children treated with acyclovir during the second week after exposure compared with 100% of children in the control group (72). In another study, 85% of children who were treated during the first week after exposure seroconverted (a fourfold or greater increase in antibody in convalescent sera), as did 79% of those treated during the second week after exposure (73). Seroconversion in the absence of clinical disease occurred more frequently in children who received acyclovir during the second week after exposure (73%) than among those who received acyclovir during the first week after exposure (9%). Thus, although acyclovir appeared to prevent or modify clinical disease in most cases, some children remained susceptible. Serologic testing is required to distinguish those children with subclinical disease who seroconverted from those children who were not infected (74). Postexposure use of acyclovir may be a less costly alternative to the use of VZIG in some susceptible persons. However, before such a strategy could be considered, additional data are needed concerning the prophylactic use of acyclovir in healthy and immunocompromised persons in all age groups. LIVE, ATTENUATED VARICELLA VIRUS VACCINE The varicella virus vaccine licensed in the United States is composed of the Oka strain of live, attenuated VZV. The Oka strain was isolated in Japan (75) in the early 1970s from vesicular fluid in a healthy child who had natural varicella and was attenuated through sequential propagation in cultures of human embryonic lung cells, embryonic guinea-pig cells, and human diploid cells (WI-38). The virus in the Oka/Merck vaccine has undergone further passage through human diploid-cell cultures (MRC-5) for a total of 31 passages. Varicella virus vaccine was first licensed for use among high-risk children in several European countries in 1984, in Japan in 1986, and in Korea in 1988. In Japan and Korea, licensure was extended to healthy children in 1989; no concerns about vaccine safety have been identified after the administration of greater than 2 million doses in these countries. Varicella virus vaccine is lyophilized, and when reconstituted as directed in the package insert and stored at room temperature for 30 minutes, the vaccine contains greater than 1,350 plaque forming units (PFUs) of Oka/Merck VZV in each 0.5-mL dose. Each 0.5-mL dose also contains 12.5 mg of hydrolyzed gelatin, trace amounts of neomycin and fetal bovine serum, 25 mg of sucrose, and trace residual components of MRC-5 cells (including DNA and protein). The vaccine does not contain preservatives. Since 1981, a total of 9,454 healthy children and 1,648 healthy adolescents and adults have received several formulations of the Oka/Merck vaccine as part of clinical trials conducted in the United States (76-83). The occurrence of serious adverse events has been minimal (see Vaccine-Associated Adverse Events). Antibody responses have been measured by gpELISA. Immunogenicity The seroconversion rate (gpELISA greater than 0.3 U) after one dose of vaccine among 6,889 susceptible children 12 months-12 years of age was demonstrated to be 97%; 76% of these children achieved antibody titers of gpELISA greater than or equal to 5 U (Merck and Company, Inc., unpublished data). Persistence of antibody (i.e., IgG greater than 5 by FAMA) measured yearly for 4 years after vaccination was consistently high (i.e., greater than 90%) in children vaccinated at 12, 13, 14, 15, 16-23, 24-36, or 48-144 months of age, although the numbers of children tested decreased considerably as the length of time since vaccination increased. Six years after vaccination at ages ranging from 1 to 12 years, 35 children had no decrease in antibody titers (84). In Japan, antibodies to VZV were present in 97% of children 7-10 years after vaccination, and titers were comparable to those in children who had natural varicella infection 7-10 years earlier (85). A subsequent 20-year follow-up study revealed that antibody levels were higher than those observed 10 years earlier (86). These higher levels might have resulted from asymptomatic boosting of vaccine-induced immunity by exposure to wild-type VZV, because less than 20% of children in Japan were vaccinated during 1991-1993. Among persons greater than or equal to 13 years of age, 78% of vaccinees seroconverted after the first dose of varicella virus vaccine, and 99% seroconverted after a second dose, which was administered 4-8 weeks later (Merck and Company, Inc., unpublished data); the proportion of vaccinees who seroconverted did not differ by age. Detectable antibody levels have persisted for at least 1 year in 97% of adolescents and adults who were administered two doses of vaccine 4-8 weeks apart. Efficacy and Breakthrough Infections In clinical trials, the vaccine has proven to be effective for greater than 10 years in preventing varicella. However, breakthrough infections (i.e., cases of varicella that occur in some vaccinated persons following exposure to wild-type virus) can occur, usually resulting in mild illness. In a double-blind, placebo-controlled trial using vaccine that contained 17,430 PFUs, efficacy among children 1-14 years of age was 100% after the first varicella season and 96% after the second season. On the basis of a long-term evaluation of a subset of vaccinees whose vaccination status was revealed after the second year following vaccination, efficacy was an estimated 95% after 7 years (77). A controlled efficacy trial was not conducted for adults. Data from all trials in which vaccinees of all ages were actively followed for up to 9 years indicated that varicella developed in less than 1%-4.4% of vaccinees per year, depending on vaccine lot and time interval since vaccination (Merck and Company, Inc., unpublished data). Active and passive surveillance data collected during 6 years of follow-up have indicated that each year, varicella developed in 2.1%-3.6% of the 4,142 children who received earlier lots of vaccine containing 1,000-1,625 PFUs of attenuated virus. Natural varicella attack rates in children 1-9 years of age are an estimated 8.3%-9.1%; thus, these data represent an estimated 67% (range: 57%-77%) decrease from the total number of expected cases. For up to 3 years of follow-up, varicella developed in 0.2%-1% of 1,164 children who received the current vaccine containing 2,900-9,000 PFUs per year, representing an average 93% decrease from expected rates. The attack rate after household exposure for a subset of 259 persons who received early lots of vaccine decreased by 77% compared with the expected attack rate for unvaccinated persons (attack rate: 20% and 87%, respectively) (45,64; Merck and Company, Inc., unpublished data). In these clinical trials, varicella subsequently developed in substantially fewer children who had postvaccination gpELISA titers of greater than or equal to 5 U varicella than those children who had postvaccination gpELISA titers of less than 5 U. Varicella is substantially less severe among vaccinated persons than unvaccinated persons, who usually have fever and several hundred vesicular lesions (87). For vaccinees in whom varicella has developed, the median number of skin lesions has been less than 50 (88,89; Merck and Company, Inc., unpublished data). In addition, most vaccinees have been afebrile, have had fewer vesicular lesions, and have had shorter duration of illness than unvaccinated persons. Illnesses associated with vaccine failure are attenuated and have not increased in severity during the 7-10 years of follow-up study. The rate of disease transmission from vaccinees in whom varicella develops is low for children but has not been studied in adults. In 10 different trials conducted from 1981 through 1989 involving 2,141 vaccinated children, breakthrough infections occurred in 78 vaccinated children during the 1-8 year follow-up period of active surveillance, resulting in secondary transmission to 11 (12.2%) of their 90 vaccinated siblings (89). Illness was mild in both index and secondary case-patients. Transmission to a susceptible mother from a vaccinated child in whom breakthrough disease occurred also has been reported (Merck and Company, Inc., unpublished data). Varicella virus vaccine provides 70%-90% protection against infection and 95% protection against severe disease for 7-10 years after vaccination. Data are insufficient to evaluate the extent of the protection provided by varicella vaccination against serious complications from varicella (e.g., bacterial infections of skin lesions, pneumonia, and encephalitis) in persons of all ages. However, serious complications are anticipated to be reduced, because data indicate attenuation of common manifestations of disease in vaccinees. Current data concerning vaccine efficacy and persistence of antibody in vaccinees are based on research that has been conducted when natural VZV infection has been highly prevalent and has not been affected by wide use of the vaccine. Thus, the extent to which the protection provided by vaccination has been increased by boosting from exposure to natural virus and whether longer term immunity may wane as the circulation of natural VZV decreases are unknown. Transmission of Vaccine Virus Available data suggest that healthy children are unlikely to transmit vaccine virus to susceptible contacts, but that risk for transmission from vaccinees who are immunocompromised is higher and may be associated with occurrence of rash following vaccination. Risk for transmission of vaccine virus was assessed in siblings of vaccinated children who received placebo themselves (76). During the 8 weeks following vaccination, six (1%) of 439 placebo recipients seroconverted without rash; their vaccinated siblings also had no rash. Among three of the six children who seroconverted, serologic data suggested that the preparations administered to the assigned vaccine and placebo recipients were mistakenly switched. In addition, varicella developed in three other placebo recipients during months of high varicella incidence (i.e., from December through June); one recipient had had an exposure to natural varicella at school. In another study, no evidence of transmission of vaccine virus was found after vaccinating 37 healthy siblings of 30 immunocompromised children (90). None of the immunocompromised children had rash or demonstrated evidence of humoral or cell-mediated immune responses. Higher risk for transmission of vaccine virus has been documented among children who have both rash following vaccination and leukemia. Data from one study indicated that varicella virus vaccine infection occurred in 15 (17%) of 88 exposed, healthy siblings of leukemic vaccine recipients; mild rash developed in 11 siblings (91). In one family, tertiary transmission to a second healthy sibling occurred, with rash developing 18 days after rash onset in the secondary case-patient and 33 days after rash onset in the leukemic child. Both healthy siblings had mild rash (i.e., 11 and 40 lesions, respectively), and vaccine virus was isolated from all three case-patients. These data suggest that healthy, vaccinated persons have a minimal risk for transmitting vaccine virus to their contacts; this risk may be higher in vaccinees in whom a varicella-like rash develops following vaccination. In clinical trials, nonlocalized rash developed in 3.8% of children and 5.5% of adolescents and adults (median: five lesions) after the first injection and 0.9% of adolescents and adults after the second injection. Herpes Zoster Following Vaccination The incidence of herpes zoster after varicella vaccination among otherwise healthy children is approximately 18 per 100,000 person years of follow-up (Merck and Company, Inc., unpublished data). A population-based study indicated that the incidence of herpes zoster after natural varicella infection among healthy children was 77 per 100,000 person years. However, these two rates should be compared cautiously, because the latter rate was based on a larger pediatric population that was monitored for a longer period of time than were the vaccinees (92,93). One case of herpes zoster has been reported among adult vaccinees, resulting in an incidence of 12.8 per 100,000 person years. Although unknown, the rate of herpes zoster in unvaccinated adults is expected to be higher than that in adult vaccinees. All of the vaccinees' illnesses were mild and without complications. Wild-type virus was identified in one vaccinated child and one vaccinated adult by using restriction endonuclease analysis in cultures from vesicles, which suggests that some herpes zoster cases in vaccinees may result from antecedent natural varicella infection (Merck and Company, Inc., unpublished data;94). Vaccine as Postexposure Prophylaxis No data exist regarding postexposure efficacy of the current varicella virus vaccine. Postexposure prophylaxis of children using previous formulations of varicella virus vaccine has been conducted in two controlled studies. In Japan (95) and the United States (79), protective efficacy was greater than or equal to 90% when children were vaccinated within 3 days of exposure. Cost Benefit of Vaccine A recent cost-effectiveness study (96) was performed using current estimates of morbidity and mortality (CDC, unpublished data), mathematical modeling of the projected impact of vaccination (97), and current direct and indirect costs. Unlike a previous study published in 1985 (98), the recent analysis accounted for potential changes in the frequency and severity of varicella-related complications resulting from expected changes in the epidemiology and age distribution of varicella following widespread use of varicella virus vaccine. Additional efficacy data for 1985-1993 were available, and empiric data on medical utilization and costs of work-loss resulting from varicella were used. The results of this study, which were determined using an estimated cost of $35 per dose of vaccine and $5 for vaccine administration, indicated a savings of $5.40 for each dollar spent on routine vaccination of preschool-age children when direct and indirect costs were considered. When only direct medical costs were considered, the benefit-cost ratio was 0.90:1. Benefit-cost ratios were only slightly lower when lower estimates of the short-term and long-term effectiveness of the vaccine were used. DISTRIBUTION, HANDLING, AND STORAGE OF VACCINE To maintain potency, the lyophilized vaccine must be stored frozen at an average temperature of less than or equal to 5 F (less than or equal to -15 C). Household freezers manufactured since the mid-1980s are designed to maintain temperatures from -4 F (-20 C) to 5 F (-15 C). When tested, VARIVAXM has remained stable in frost-free refrigerators. Refrigerators with ice compartments that are either not tightly enclosed or enclosed with unsealed, uninsulated doors (e.g., small, dormitory-style refrigerators) may not meet temperature requirements. Regardless of the type of freezer, providers should check the adequacy of their freezer by verifying its temperature before obtaining vaccine. The diluent should be stored separately either at room temperature or in the refrigerator. The vaccine should be reconstituted according to the directions in the package insert and only with the diluent supplied with the vaccine, which does not contain preservative or other antiviral substances that could inactivate the vaccine virus. Once reconstituted, the vaccine should be used immediately to minimize loss of potency. The vaccine should be discarded if not used within 30 minutes after reconstitution. Handling of Vaccine Within a Clinic and for Clinics That do not Have Adequate Facilities to Store Vaccine When an immunization session is being held at a site distant from the freezer in which the vaccine is stored, the needed number of vaccine vials for the immunization session should be stored in a suitable container (i.e., the original shipping container or a comparable container with a properly fitting lid) with an adequate quantity of dry ice (i.e., a minimum of 6 lbs/box), so that dry ice would remain if any unreconstituted vaccine must be returned to the freezer. Dry ice, when placed in a suitable container, will maintain a temperature of 5 F (-15 C) or colder. When optimal handling conditions are not feasible because of the location of the freezer storage area or concern for security of the room where vaccines are administered within a clinic, or when vaccine must be transported to a clinic site distant from the freezer-storage area, minimal potency can be maintained if varicella virus vaccine is stored continuously for up to 72 hours at temperatures of 36-46 F (2-8 C). This vaccine should be discarded if not used within 72 hours of placing it into storage. * Minimizing Wastage of Vaccine Vaccine wastage can be minimized by accurately determining the number of doses needed for clinics that do not have adequate freezer-storage facilities. To ensure maximal vaccine potency, smaller shipments of vaccine should be ordered more frequently -- preferably at least once every 3 months. Vaccine lots with a longer expiration period (i.e., greater than 12 months to expiration) should be selected for use in clinics that do not have adequate facilities to store vaccine. Transfer of Vaccine Between Clinic Sites When transferring vaccine between clinic sites is required (e.g., when supply must be adjusted), the vaccine should be packed in the manufacturer's shipping container or a container with comparable insulating qualities using appropriate quantities of dry ice (e.g., a minimum of 6 lbs/box). Residual dry ice should be available at the receiving site. If dry ice is not available, the vaccine should be discarded unless a temperature recorder has been included in the transport box; if the temperature has been less than or equal to 36-46 F (less than or equal to 2-8 C) for up to 72 hours, the vaccine can be used within 72 hours of removal from the freezer-storage area. RECOMMENDATIONS FOR THE USE OF VARICELLA VIRUS VACCINE Persons less than 13 Years of Age Varicella virus vaccine has been approved for use among healthy children 12 months-12 years of age. Children in this age group should receive one 0.5-mL dose of vaccine subcutaneously. Children who have a reliable history of varicella are considered immune, and those who do not have such a history or who have an uncertain history of varicella are considered susceptible. Serologic testing of children before vaccination is not warranted because a) most children 12 months-12 years of age who do not have a clinical history of varicella are susceptible and b) the vaccine is well tolerated in seropositive persons. 12-18 Months of Age. All children should be routinely vaccinated at 12-18 months of age. Varicella virus vaccine may be administered to all children at this age -- regardless of a history of varicella; however, vaccination is not necessary for children who have reliable histories of varicella. Varicella virus vaccine preferably should be administered routinely to children at the same time as measles-mumps-rubella (MMR) vaccine. Varicella virus vaccine is safe and effective in healthy children greater than or equal to 12 months of age when administered at the same time as MMR vaccine at separate sites and with separate syringes or when administered separately greater than or equal to 30 days apart. The number and types of adverse events in children who have received VARIVAX and MMR concurrently have not differed from those in children who have been administered the vaccines at different visits (Merck and Company, Inc., unpublished data). Data concerning the effect of simultaneous administration of VARIVAX with various combinations of MMR-, diphtheria and tetanus toxoids and pertussis (DTP)-, and Haemophilus influenzae type b (Hib)-containing vaccines have not yet been published. However, data regarding simultaneous administration of an investigational quadrivalent vaccine containing varicella (MMRII VM) with diphtheria and tetanus toxoids and acellular pertussis (DTaP) and Hib vaccines suggest that no notable interactions exist between varicella and any other vaccines that are routinely administered to young children (e.g., measles, mumps, rubella, diphtheria, tetanus, pertussis, and Haemophilus influenzae type b vaccines). Furthermore, the simultaneous administration of most widely used live, attenuated and inactivated vaccines has not resulted in impaired antibody response or an increased rate of adverse events. Therefore, varicella virus vaccine may be administered simultaneously with all of the vaccines recommended for children 12-18 months of age. Simultaneous administration is particularly important when health-care providers anticipate that, because of certain factors (e.g., previously missed vaccination opportunities), a child may not return for subsequent vaccination. 19 Months-12 Years of Age. Varicella vaccine is recommended for all susceptible children by their 13th birthday. After 12 years of age, natural varicella is more severe and complications are more frequent. Recently, ACIP recommended establishing a routine immunization visit at 11-12 years of age to review immunization status and to administer necessary vaccinations (99). Although vaccine may be administered at any time after 18 months of age, varicella virus vaccine should be administered to susceptible children during this routine visit. Persons greater than or equal to 13 Years of Age Varicella vaccine is approved for use among healthy adolescents and adults. Because natural VZV infection can be severe in older adolescents and adults, varicella immunity is desirable in these age groups. Persons greater than or equal to 13 years of age should be administered two 0.5-mL doses of vaccine, subcutaneously, 4-8 weeks apart. If greater than 8 weeks elapse following the first dose, the second dose can be administered without restarting the schedule. Persons greater than or equal to 13 years of age who have reliable histories of varicella are considered immune. Those who do not have such histories are considered susceptible and can be tested to determine immune status or can be vaccinated without testing. Because 71%-93% of adults who do not have a reliable history of varicella are actually immune (1,26,42,46,47), serologic testing before vaccination is likely to be cost effective for both adults and adolescents (100). Adolescents and adults should be assessed for varicella immune status, and those who are susceptible should be vaccinated. Priority should be given to vaccination of susceptible adolescents and adults who are at high risk for exposure and for transmitting disease; specific assessment efforts are targeted to these persons (Box 1). Box 1. Vaccination of persons >=13 years of age
Health-Care Workers * All susceptible health-care workers should ensure that they are immune to varicella. In health-care institutions, serologic screening of personnel who have a negative or uncertain history of varicella is likely to be cost effective. Routine testing for varicella immunity after two doses of vaccine is not necessary for the management of vaccinated health-care workers who may be exposed to varicella, because 99% of persons are seropositive after the second dose. Seroconversion, however, does not always result in full protection against disease. Testing vaccinees for seropositivity immediately after exposure to VZV is a potentially effective strategy for identifying persons who remain at risk for varicella. Prompt serologic results may be obtained using the LA test. Varicella is unlikely to develop in persons who have detectable antibody; persons who do not have such antibody can be retested in 5-6 days to determine if an anamnestic response is present, in which case development of disease is unlikely. Persons who remain susceptible may be furloughed. Alternatively, persons can be monitored daily to determine clinical status and then furloughed at the onset of manifestations of varicella. Institutional guidelines are needed for the management of exposed vaccinees who do not have detectable antibody and for persons who develop clinical varicella. More information is needed concerning the risk for transmission of vaccine virus from vaccinees in whom varicella-like rash develops following vaccination. On the basis of available data, the risk appears to be minimal, and the benefits of vaccinating susceptible health-care workers outweigh this potential risk. As a safeguard, institutions may wish to consider precautions for personnel in whom rash develops following vaccination and for other vaccinated personnel who will have contact with susceptible persons at high risk for serious complications. Vaccination should be considered for unvaccinated health-care workers who are exposed to varicella and whose immunity is not documented. However, because the protective effects of postexposure vaccination are unknown, persons vaccinated after an exposure should be managed in the manner recommended for unvaccinated persons. Household Contacts of Immunocompromised Persons Immunocompromised persons are at high risk for serious varicella infections. Disseminated disease occurs in approximately 30% of such persons who have primary infection. Vaccination of household contacts provides protection for immunocompromised persons by decreasing the likelihood that wild-type varicella virus will be introduced into the household. Vaccination of household contacts of immunocompromised persons theoretically may pose a minimal risk of transmission of vaccine virus to immunocompromised persons, although in one study, no evidence of transmission of vaccine virus was found after vaccinating 37 healthy siblings of 30 children with malignancy. Available data indicate that disease caused by vaccine virus in immunocompromised persons is milder than wild-type disease and can be treated with acyclovir. More information is needed concerning the risk for transmission of the vaccine virus from both vaccinees who have and who do not have varicella-like rash following vaccination. On the basis of available data, the benefits of vaccinating susceptible household contacts of immunocompromised persons outweigh the potential risk for transmission of vaccine virus to immunocompromised contacts. VACCINE-ASSOCIATED ADVERSE EVENTS Varicella virus vaccine has been well tolerated when administered to greater than 11,000 healthy children, adolescents, and adults during clinical trials. Inadvertent vaccination of persons immune to varicella has not resulted in an increase in adverse events. In a double-blind, placebo-controlled study of 914 healthy, susceptible children and adolescents (76), pain and redness at the injection site were the only adverse events that occurred significantly more often (p less than 0.05) in vaccine recipients than in placebo recipients. Persons 12 Months-12 Years of Age In uncontrolled clinical trials of approximately 8,900 healthy children (Merck and Company, Inc., package insert) who were administered one dose of vaccine and then monitored for up to 42 days, 14.7% developed fever (i.e., oral temperature greater than or equal to 102 F {greater than or equal to 39 C}); these febrile episodes occurred throughout the 42-day period and were usually associated with intercurrent illness. A total of 19.3% of vaccine recipients had complaints regarding the injection site (e.g., pain/soreness, swelling, erythema, rash, pruritus, hematoma, induration, and stiffness), 3.4% had a mild, varicella-like rash at the injection site consisting of a median number of two lesions and occurring at a peak of 8-19 days postvaccination, and 3.8% had a nonlocalized, varicella-like rash consisting of a median number of five lesions and occurring at a peak of 5-26 days postvaccination. Febrile seizures following vaccination occurred in less than 0.1% of children; a causal relationship has not been established. Persons greater than or equal to 13 Years of Age In uncontrolled trials of persons greater than or equal to 13 years of age, approximately 1,600 vaccinees who received one dose and 955 who received two doses of varicella vaccine were monitored for 42 days for adverse events (Merck and Company, Inc., package insert). After the first and second doses, 10.2% and 9.5% of vaccinees, respectively, developed fever (i.e., oral temperature greater than or equal to 100 F {37.7 C}); these febrile episodes occurred throughout the 42-day period and were usually associated with intercurrent illness. After one and two doses, 24.4% and 32.5% of vaccinees, respectively, had complaints regarding the injection site (e.g., soreness, swelling, erythema, rash, pruritus, hematoma, pyrexia, induration, and numbness); a varicella-like rash at the injection site consisting of a median number of two lesions and occurring at a peak of 6-20 days and 0-6 days postvaccination, respectively, developed in 3% and 1% of vaccinees, respectively; and a nonlocalized rash consisting of a median number of five lesions developed in 5.5% and 0.9% of vaccinees, respectively, and occurred at a peak of 7-21 days and 0-23 days postvaccination, respectively. Postlicensure Adverse Vaccine Events During the first 12 months following vaccine licensure, more than 2.3 million doses of vaccine were distributed in the United States. The Vaccine Adverse Events Reporting System (VAERS) and the vaccine manufacturer have received a limited number of reports of serious medical events occurring within 6 weeks after varicella virus vaccination, including encephalitis (n=4), ataxia (n=7), and erythema multiforme (n=10). Three cases of anaphylaxis have occurred within 10 minutes of vaccination. A causal relationship between the vaccine and these events has not been determined. Potential delayed or underreporting of events to VAERS may have occurred. Physicians and health-care providers are encouraged to report any suspected adverse events that occur after varicella virus vaccination (see Reporting of Adverse Events). Postmarketing surveillance for adverse events will be ongoing. Reporting of Adverse Events The National Vaccine Injury Act of 1986 (101) requires physicians and other health-care providers who administer vaccines to maintain permanent immunization records and to report occurrences of adverse events for selected vaccines. Although the Act currently does not apply to varicella virus vaccine, the same recording and reporting requirements should be followed. Serious adverse events (i.e., all events requiring medical attention), regardless of whether they are suspected to have been caused by varicella virus vaccine, should be reported to VAERS. VAERS forms and instructions are available in the FDA Drug Bulletin and the Physicians' Desk Reference or by calling the 24-hour VAERS information recording (telephone: {800} 822-7967). CONTRAINDICATIONS AND PRECAUTIONS Allergy to Vaccine Components The administration of live varicella virus vaccine rarely results in hypersensitivity. The information in the package insert should be carefully reviewed before vaccine is administered; vaccination is contraindicated for persons who have a history of anaphylactic reaction to any component of the vaccine, including gelatin. Varicella virus vaccine does not contain preservatives or egg protein -- substances that have caused hypersensitive reactions to other vaccines. Varicella virus vaccine should not be administered to persons who have a history of anaphylactic reaction to neomycin. Neomycin allergy is usually manifested as a contact dermatitis, which is a delayed-type immune response rather than anaphylaxis. For persons who experience such a response, the adverse reaction, if any, would be an erythematous, pruritic nodule or papule present 48-96 hours after vaccination. A history of contact dermatitis to neomycin is not a contraindication to receiving varicella virus vaccine. Illness Vaccination of persons who have severe illness should be postponed until recovery. The decision to delay vaccination depends on the severity of symptoms and on the etiology of the disease. Vaccine can be administered to susceptible children who have mild illnesses with or without low-grade fever (e.g., diarrhea or upper-respiratory infection) (102). Studies suggest that failure to vaccinate children with minor illnesses can impede vaccination efforts (103). Although no data exist regarding whether either varicella or live varicella virus vaccine exacerbates tuberculosis, vaccination is not recommended for persons who have untreated, active tuberculosis. Tuberculin skin testing, however, is not a prerequisite for varicella vaccination. Altered Immunity Varicella virus vaccine is not licensed for use in persons who have any malignant condition, including blood dyscrasias, leukemia, lymphomas of any type, or other malignant neoplasms affecting the bone marrow or lymphatic systems. However, vaccine is available to any physician free of charge from the manufacturer * through a research protocol (104) for use in patients who have acute lymphoblastic leukemia (ALL) who a) are 12 months-17 years of age, b) have disease that has been in remission for at least 12 continuous months, c) have a negative history of varicella disease, d) have a peripheral blood-lymphocyte count of greater than 700 cells/mm3, and e) have a platelet count of greater than 100,000 cells/mm3 within 24 hours of vaccination. The vaccine is well tolerated, immunogenic, and protective in children who meet these criteria (105-110). The most common reaction to the vaccine in patients who have ALL is a mild to moderate varicella-like rash (i.e., two to 200 lesions), which occurs in approximately 5% of children who have completed their chemotherapy before vaccination and 40% of vaccinees on maintenance chemotherapy (106). Varicella virus vaccine should not be administered to persons who have primary or acquired immunodeficiency, including immunosuppression associated with acquired immunodeficiency syndrome (AIDS) or other clinical manifestations of human immunodeficiency virus (HIV) infections, cellular immunodeficiencies, hypogammaglobulinemia, and dysgammaglobulinemia. The use of varicella virus vaccine in persons who are infected with HIV has not been studied; therefore, vaccination of these persons is not recommended, although routine screening for HIV before vaccination also is not recommended. The use of varicella virus vaccine in HIV-infected children is being investigated. If inadvertent vaccination of HIV-infected persons results in clinical disease, the use of acyclovir may modify the severity of disease. Varicella virus vaccine should not be administered to persons who have a family history of congenital or hereditary immunodeficiency in first-degree relatives (e.g., parents and siblings) unless the immune competence of the potential vaccine recipient has been clinically substantiated or verified by a laboratory. Varicella virus vaccine should not be administered to persons receiving immunosuppressive therapy -- except children who have ALL in remission, as previously described. Such persons are more susceptible to infections than healthy persons. Administration of live, attenuated varicella virus vaccine can result in a more extensive vaccine-associated rash or disseminated disease in persons receiving immunosuppressive doses of corticosteroids (111). This contraindication does not apply to persons who are receiving corticosteroid-replacement therapy. Children Who Have Conditions That Require Steroid Therapy No data have been published concerning whether susceptible children receiving only inhaled doses of steroids can be vaccinated safely. However, most experts concur, on the basis of clinical experience, that vaccination of these children is safe. Susceptible children who are receiving systemic steroids for certain conditions (e.g., asthma) and who are not otherwise immunocompromised can be vaccinated if they are receiving less than 2 mg/kg of body weight or a total of 20 mg/day of prednisone or its equivalent. Antibody status should be assessed 6 weeks postvaccination, and children who have not seroconverted should be revaccinated. Some experts suggest withholding steroids for 2-3 weeks following vaccination when possible. Data from one study conducted in Japan indicated that children taking steroids for nephrosis were vaccinated safely when the steroids were suspended for 1-2 weeks before vaccination, although no serious reactions occurred among children vaccinated when steroid therapy was not suspended (112). Children who are receiving high doses of systemic steroids (i.e., greater than or equal to 2 mg/kg prednisone) for greater than or equal to 2 weeks may be vaccinated after steroid therapy has been discontinued for at least 3 months in accordance with the general recommendations for the use of live-virus vaccines (113); however, withholding steroids for at least 1 month before varicella vaccination is probably sufficient. Exposure of Immunocompromised Persons to Vaccinees Healthy persons in whom varicella-like rash develops following vaccination appear to have a minimal risk for transmission of vaccine virus to their close contacts (e.g., family members). Seroconversion has been documented in healthy siblings of healthy vaccinees in whom rash did not develop, although such an occurrence is rare (76). Vaccinees in whom vaccine-related rash develops, particularly health-care workers and household contacts of immunocompromised persons, should avoid contact with susceptible persons who are at high risk for severe complications. If a susceptible, immunocompromised person is inadvertently exposed to a person who has a vaccine-related rash, VZIG need not be administered because disease associated with this type of transmission is expected to be mild. Recent Administration of Blood, Plasma, or Immune Globulin Although passively acquired antibody is known to interfere with response to measles and rubella vaccines (114), the effect of the administration of immune globulin (IG) on the response to varicella virus vaccine is unknown. The duration of interference with the response to measles vaccination depends on the dosage and ranges from 3-11 months. Because of the potential inhibition of the response to varicella vaccination by passively transferred antibodies, varicella virus vaccine should not be administered for at least 5 months after administration of blood (except washed red blood cells), plasma, IG, or VZIG (113). In addition, IG and VZIG should not be administered for 3 weeks after vaccination unless the benefits exceed those of vaccination. In such cases, the vaccinee should either be revaccinated 5 months later or tested for immunity 6 months later and then revaccinated if seronegative. Use of Salicylates No adverse events associated with the use of salicylates after varicella vaccination have been reported. However, the vaccine manufacturer recommends that vaccine recipients avoid using salicylates for 6 weeks after receiving varicella virus vaccine because of the association between aspirin use and Reye syndrome following varicella. Vaccination with subsequent close monitoring should be considered for children who have rheumatoid arthritis or other conditions requiring theraputic aspirin because the risk for serious complications associated with aspirin is likely to be greater in children in whom natural varicella disease develops than in children who receive the vaccine containing attenuated VZV. No association has been documented between Reye syndrome and analgesics or antipyretics that do not contain salicylic acid. Pregnancy The effects of the varicella virus vaccine on the fetus are unknown; therefore, pregnant women should not be vaccinated. Nonpregnant women who are vaccinated should avoid becoming pregnant for 1 month following each injection. For susceptible persons, having a pregnant household member is not a contraindication to vaccination. If a pregnant woman is vaccinated or becomes pregnant within 1 month of vaccination, she should be counseled about potential effects on the fetus. Wild-type varicella poses only a very small risk to the fetus (see Prenatal and Perinatal Exposure). Because the virulence of the attenuated virus used in the vaccine is less than that of the wild-type virus, the risk to the fetus, if any, should be even lower. In most circumstances, the decision to terminate a pregnancy should not be based on whether vaccine was administered during pregnancy. The manufacturer, in collaboration with CDC, has established the VARIVAX Pregnancy Registry to monitor the maternal-fetal outcomes of pregnant women who are inadvertently administered varicella virus vaccine 3 months before or at any time during pregnancy (telephone: {800} 986-8999) (115). Nursing Mothers Whether attenuated vaccine VZV is excreted in human milk and, if so, whether the infant could be infected are not known. Most live vaccines have not been demonstrated to be secreted in breast milk. Attenuated rubella vaccine virus has been detected in breast milk but has produced only asymptomatic infection in the nursing infant. Therefore, varicella virus vaccine may be considered for a nursing mother. USE OF VZIG FOR POSTEXPOSURE PROPHYLAXIS Studies conducted in 1969 indicated that zoster immune globulin (ZIG) (prepared from patients recovering from herpes zoster) prevented clinical varicella in susceptible, healthy children if administered within 72 hours of exposure (116). ZIG also lowered attack rates among immunocompromised persons if administered no later than 96 hours after exposure (116). VZIG (prepared from plasma obtained from healthy, volunteer blood donors who are identified by routine screening to have high antibody titers to VZV) became available in 1978. Both serologic and clinical evaluations have demonstrated that the product is equivalent to ZIG in preventing or modifying clinical illness in susceptible, immunocompromised persons who are exposed to varicella. VZIG is a sterile, 10%-18% solution of the globulin fraction of human plasma, primarily immunoglobulin G (IgG) in 0.3 M glycine as a stabilizer and 1:10,000 thimerosal as a preservative. VZIG is prepared by using Cohn cold ethanol precipitation, which eliminates hepatitis B virus, HIV, and other known infectious agents from the product. VZIG prepared using an additional viral-inactivation step (the solvent detergent treatment) has recently become available. Supply VZIG is produced by the United States Biologics Laboratories (Massachusetts) and is distributed by American Red Cross regional distribution centers and service areas (Appendix). In Massachusetts, VZIG is distributed by the Massachusetts Department of Public Health. Administration VZIG provides maximum benefit when administered as soon as possible after the presumed exposure, but it may be effective if administered as late as 96 hours after exposure. The effectiveness of VZIG when administered 96 hours after initial exposure has not been evaluated. VZIG is not recommended for vaccinated persons who were previously seropositive and found to be seronegative following exposure because such persons would be expected to have a mild case; no further benefit would be gained by administering VZIG. VZIG has not been proven to be useful in treating clinical varicella or herpes zoster or in preventing disseminated zoster and is not recommended for such use. The duration of protection that is provided after administration of VZIG is unknown, but protection should last at least one half-life of the IG (i.e., approximately 3 weeks). Susceptible persons at high risk for whom varicella vaccination is contraindicated and who are again exposed greater than or equal to 3 weeks after a dose of VZIG should receive another full dose of VZIG. Dosage VZIG is supplied in two different dosages -- the 125-U vial and the 625-U vial. The recommended dose is 125 U/10 kg (22 lbs) of body weight, up to a maximum of 625 U. The minimum dose is 125 U; fractional doses are not recommended. VZIG administration has not been evaluated as a prophylactic measure in healthy or immunocompromised adults. Therefore, the appropriate dose for prophylaxis in adults cannot be calculated. However, 625 U should be sufficient to modify or prevent infection in healthy adults. Higher doses may be necessary for immunocompromised adults. VZIG should be administered intramuscularly as directed by the manufacturer. VZIG should never be administered intravenously. Indications for the Use of VZIG The decision to administer VZIG to a person exposed to varicella should be based on a) whether the patient is susceptible (either by having a negative history of varicella or by lacking documentation of vaccination), b) whether the exposure is likely to result in infection, and c) whether the patient is at greater risk for complications than the general population. VZIG is costly (i.e., approximately $90 per 125-U vial or $400 for persons greater than 40 kg {88 lbs} body weight) and only provides temporary protection. The long-term effects of VZIG on immunity and on the occurrence of herpes zoster are unknown. For immunocompromised persons, an accurate history of varicella should be obtained before determining immune status and whether to administer VZIG. Immunocompromised persons who do not have histories of disease, but who have low levels of antibody detected by the most sensitive assays, have contracted varicella. Presumably, the low levels of antibody in most of these patients were passively acquired from recent transfusions of blood, blood derivatives, or blood products containing antibody. Both healthy and immunocompromised children and adults who have positive histories of varicella (except for bone-marrow transplant recipients) can be considered immune (see Recommendations for the Use of Varicella Virus Vaccine). However, varicella may develop in some infants after exposure despite the presence of detectable antibody, although in most circumstances such illness is less severe than the illness occurring in infants who do not have detectable antibody. Therefore, sensitive assays may not be useful in assessing whether clinical disease will develop in neonates or young infants exposed to varicella. The association between positive histories of varicella in bone-marrow donors and susceptibility to varicella in recipients following transplants has not been adequately studied. Thus, persons who receive bone-marrow transplants should be considered susceptible -- regardless of prior history of varicella or varicella vaccination in themselves or in their donors. Bone-marrow recipients in whom varicella or herpes zoster develops following transplantation should subsequently be considered immune. Types of Exposure Several types of exposure can place susceptible persons at risk for varicella. Direct contact exposure is defined as greater than 1 hour of direct contact with an infectious person while indoors; substantial exposure for hospital contacts consists of sharing the same hospital room with an infectious patient or prolonged, direct, face-to-face contact with an infectious person (e.g., health-care workers). Brief contacts with an infectious person (e.g., contact with x-ray technicians or housekeeping personnel) are less likely to result in VZV transmission than are more prolonged contacts. Persons with continuous exposure to household members who have varicella are at greatest risk for infection. Varicella develops in approximately 90% of susceptible household contacts. Following household exposure, attack rates among immunocompromised children administered VZIG are 33%-50%. Data are not available for immunocompromised, susceptible persons who were not administered VZIG. The risk for varicella following close contact (e.g., contact with playmates) or hospital exposure is approximately 20% of the risk occurring from household exposure. The attack rate in healthy neonates who are exposed in utero within 5 days of delivery and administered VZIG after birth is 30%- 40%, which is not substantially different from rates reported for neonates who are similarly exposed but not treated with VZIG. However, the occurrence of complications and fatal outcomes is substantially lower for neonates who are treated with VZIG than for those who are not. RECOMMENDATIONS FOR THE USE OF VZIG Persons less than 13 Years of Age Immunocompromised children. VZIG primarily is used for passive immunization of susceptible, immunocompromised children after substantial exposure to varicella or herpes zoster -- including children who a) have primary and acquired immune-deficiency disorders, b) have neoplastic diseases, and c) are receiving immunosuppressive treatment. Data are limited regarding whether routine therapy with intravenous IG yields the persistence of a sufficient amount of passively acquired VZV antibody to protect susceptible, immunocompromised persons who become exposed to VZV. Data from one study indicated that three of 17 HIV-infected children in whom varicella developed were receiving regular intravenous IG and had detectable antibody against varicella (117). One of the three children had been treated with VZIG after two previous exposures to varicella that did not result in disease but was not administered VZIG after an unrecognized exposure, which resulted in varicella. To ensure protection against severe disease, immunocompromised persons receiving intravenous IG should be administered VZIG if exposed to wild-type VZV. Neonates whose mothers become infected with varicella shortly before delivery. VZIG is indicated for neonates whose mothers have signs and symptoms of varicella within 5 days before and 2 days after delivery. VZIG is probably not necessary for neonates whose mothers have signs and symptoms of varicella greater than 5 days before delivery, because those infants should be protected from severe varicella by transplacentally acquired maternal antibody. No evidence exists suggesting that infants born to mothers in whom varicella develops greater than 48 hours after delivery are at increased risk for serious complications (e.g., pneumonia or death). Postnatal exposure of neonates. Transmission of varicella in the hospital nursery is rare because most neonates are protected by maternal antibody. Premature infants who have substantial postnatal exposure should be evaluated on an individual basis. The risk for complications of postnatally acquired varicella in premature infants is unknown. Because the immune systems of premature infants may be compromised, administration of VZIG to those who are exposed and born to susceptible mothers may be prudent. These infants should be considered at risk for as long as they are hospitalized. Premature infants who are less than 28 weeks' gestation or who weigh less than or equal to 1,000 g at birth who are exposed to VZV should receive VZIG, regardless of maternal history, because such infants may not have acquired maternal antibody. Most premature infants of greater than or equal to 28 weeks' gestation born to immune mothers have enough acquired maternal antibody to protect them from severe disease and complications. Although infants are at higher risk for serious and fatal complications than are older children, the risk for healthy, full-term infants who develop varicella following postnatal exposure is substantially less than for infants whose mothers were infected 5 days before to 2 days after delivery. VZIG is not recommended for healthy, full-term infants who are exposed postnatally, even if their mothers have no history of varicella infection. Persons greater than or equal to 13 Years of Age Rates of complications and death for immunocompromised adolescents and adults in whom varicella develops are higher than those for healthy adolescents and adults. To prevent complications, immunocompromised persons who are considered susceptible and who have had substantial exposure to varicella should receive VZIG. The rationale for the use of VZIG among adolescent and adult patients routinely treated with intravenous IG (IVIG) is equivalent to that for the use of VZIG among immunocompromised children (see Immunocompromised Children). Varicella is usually more severe in otherwise healthy adolescents or adults than in healthy children. The decision to administer VZIG to susceptible, healthy adolescents and adults should be made on an individual basis. The objective of using VZIG among healthy adolescents and adults is to modify, rather than to prevent, illness with the hope of inducing lifetime immunity. When deciding whether to administer VZIG, clinicians should consider the patients' health status, the type of exposure, and the likelihood of previous varicella infection. Adults who were older siblings in large families or whose children have had varicella likely are immune. If, after careful evaluation, a healthy, unvaccinated adolescent or adult who has had substantial exposure is determined as being susceptible, VZIG can be considered. If varicella is prevented through the use of VZIG, vaccination should be offered later. As the use of varicella virus vaccine becomes widespread, the demand for VZIG should decrease. Pregnant women. Pregnant women should be evaluated in the same manner as other adults; however, because such women are at higher risk for severe varicella and complications (8,13,118), VZIG should be strongly considered for susceptible pregnant women who have been exposed. Administration of VZIG to susceptible, pregnant women has not been found to prevent viremia, fetal infection, congenital varicella syndrome, or neonatal varicella. Thus, the primary indication for VZIG in pregnant women is to prevent complications of varicella in the mother, rather than to protect the fetus. VZIG may extend the incubation period of the virus from 10-21 days to greater than or equal to 28 days. Neonates born to mothers who have signs and symptoms of varicella within 5 days preceding or 2 days after delivery should receive VZIG -- regardless of whether the mother received VZIG. Hospital personnel. Varicella virus vaccine is recommended for all susceptible hospital personnel; widespread use of the vaccine should limit the need for VZIG in this setting. If exposed, hospital personnel who have negative or uncertain histories of varicella and no history of vaccination should be evaluated in the same manner as other adults. In addition, types of exposure and histories of prior exposure to patients with varicella should be considered before administration of VZIG. Serologic testing also may help in assessing whether to administer VZIG and in determining whether work restrictions are necessary during the incubation period. In general, the same control measures apply -- regardless of whether susceptible personnel or patients receive VZIG. Because VZIG can prolong the incubation period of the virus, the period of removal from direct patient contact should be extended at least 1 week. VZIG-ASSOCIATED ADVERSE EVENTS AND PRECAUTIONS The most frequent adverse reaction following VZIG administration is local discomfort at the injection site. Pain, redness, and swelling occur at the injection site in approximately 1% of persons. Less frequent adverse events include gastrointestinal symptoms, malaise, headache, rash, and respiratory symptoms, which occur in approximately 0.2% of recipients. Severe events, such as angioneurotic edema and anaphylactic shock, are rare (i.e., occurring in approximately less than 0.1% of recipients). VZIG may be indicated for patients who have severe thrombocytopenia or any other coagulation disorder that would ordinarily be a contraindication to intramuscular injections. In this circumstance, the expected benefits of administering this biologic usually outweigh the risks. FUTURE CONCERNS As the use of varicella virus vaccine increases, the epidemiologic features of the disease are expected to change. The circulation of wild-type VZV is not currently affected by the use of the vaccine. Under present conditions, antibody to varicella has been demonstrated to persist for 6-10 years following vaccination in children; however, the extent to which longer-term immunity may wane is unknown. The importance of immunologic boosting following exogenous reexposure to VZV to the long-term persistence of both humoral and cell-mediated immunity to varicella is unknown. The significance of the loss of natural boosting in relation to the incidence of herpes zoster in persons who were vaccinated or exposed to wild-type virus is also unknown. Increased antibody levels have been observed in vaccinees following exposure to natural varicella; however, opportunities for immunologic boosting will be greatly reduced with widespread use of the vaccine. Revaccination 4-6 years after initial vaccination also has resulted in a boost in antibody levels. In addition, the changes in epidemiology of varicella resulting from the widespread use of vaccine in younger children are expected to decrease the circulation of wild-type virus and may establish a population of older children who were neither vaccinated nor exposed to wild-type virus, eventually resulting in a population of adults who are susceptible to varicella. School requirements for varicella immunization are possible mechanisms to ultimately prevent an increase in the population of susceptible adults. To foster more rapid control of varicella and achieve high immunity levels, state legislatures may consider including varicella vaccination in their requirements for entry into school and Head Start or day care programs. Before instituting these or other such requirements, the distribution of and access to varicella virus vaccine should be determined as adequate to accomplish universal vaccination of those children subjected to the requirements. The need for any changes in vaccine use will be determined by both postmarketing surveillance (conducted by the manufacturer of the vaccine) and ongoing surveillance (conducted by CDC). Presently, only 4%-5% of expected cases of varicella are reported annually to NNDSS. Enhanced surveillance, focused on improvement in the proportion of cases reported and the type of information obtained (including vaccination status), is needed to monitor the impact of vaccination on the incidence of varicella, the age distribution and other demographic features of infected persons, and the associated morbidity and mortality. Several active-surveillance sites have been established to provide this information until the widespread use of the vaccine reduces incidence to a manageable level and varicella becomes a nationally reportable disease. Acknowledgment The authors thank the following former members of the Advisory Committee on Immunization Practices and the associated liaison groups who were members of the Varicella Working Group: Barbara A. DeBuono, M.D., State of New York Department of Health, New York, NY; Kathryn M. Edwards, M.D., Vanderbilt University School of Medicine, Nashville, TN; Anne A. Gershon, M.D., Columbia University College of Physicians and Surgeons, New York, NY; Caroline B. Hall, M.D., American Academy of Pediatrics, Rochester, NY; Edward A. Mortimer, Jr., M.D., American Medical Association, Cleveland, OH; Georges Peter, M.D., American Academy of Pediatrics, Providence, RI; and Ronald C. Van Buren, M.D., American Academy of Family Physicians, Columbus, OH. References
Figure_1 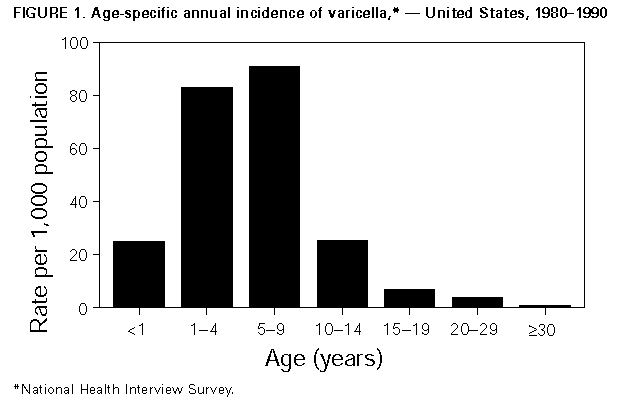 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|