 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. U.S. Public Health Service Guidelines for Testing and Counseling Blood and Plasma Donors for Human Immunodeficiency Virus Type 1 AntigenConsultants Celso Bianco, M.D. Council of Community Blood Centers New York, NY Michael Busch, M.D., Ph.D. Irwin Memorial Blood Center San Francisco, CA Richard Davey, M.D. American Red Cross Arlington, VA Steven Kleinman, M.D. American Association of Blood Banks Los Angeles, CA Susan Stramer, Ph.D. American Red Cross Atlanta, GA The following CDC staff members prepared this report: Eve M. Lackritz, M.D. Robert S. Janssen, M.D. Helene D. Gayle, M.D., M.P.H. Division of HIV/AIDS Prevention National Center for Prevention Services Charles A. Schable, M.S. Harold W. Jaffe, M.D. Division of AIDS, STD, and TB Laboratory Research National Center for Infectious Diseases in collaboration with Jay S. Epstein, M.D. Paul A. Mied, Ph.D. Sharon J. Geyer, Ph.D. Office of Blood Research and Review Center for Biologics Evaluation and Research Food and Drug Administration U.S. Public Health Service Guidelines for Testing and Counseling Blood and Plasma Donors for Human Immunodeficiency Virus Type 1 Antigen Summary The Public Health Service (PHS) has recommended a multifaceted approach to blood safety in the United States that includes stringent donor selection practices and the use of screening tests. Blood donations in the United States have been screened for antibody to human immunodeficiency virus type 1 (HIV-1) since March 1985 and type 2 (HIV-2) since June 1992. An estimated one in 450,000 to one in 660,000 donations per year (i.e.,18-27 donations) are infectious for HIV but are not detected by currently available screening tests. Because maintaining a safe blood supply is a public health priority, the Food and Drug Administration (FDA) recommended in August 1995 that all donated blood and plasma also be screened for HIV-1 p24 antigen, effective within 3 months of licensure of a test labeled for such use. Donor screening for p24 antigen is expected to reduce the number of otherwise undetected infectious donations by approximately 25% per year. Routine testing for p24 antigen in settings other than blood and plasma centers as a method for diagnosing HIV infection is discouraged because the estimated average time from detection of p24 antigen to detection of HIV antibody is 6 days, and not all recently infected persons have detectable levels of p24 antigen. Among children greater than or equal to 18 months of age and adults, diagnostic testing for HIV infection, including confirmatory testing, should routinely be performed with FDA-licensed assays for antibodies to HIV-1; p24-antigen tests alone should not be used for diagnosing HIV infection. This report provides PHS guidelines for a) interpreting p24-antigen-assay results, b) counseling and follow-up of blood donors who have positive or indeterminate p24-antigen-test results, and c) using p24-antigen testing in settings other than blood banks. INTRODUCTION In the United States, the implementation of antibody testing in 1985 of all donated blood for human immunodeficiency virus type 1 (HIV-1) resulted in a substantial decrease in the transmission of HIV through blood transfusions (1,2). To further decrease the risk for transmission of HIV by transfusion, the testing of all blood donations with a combination antibody test for HIV-1 and HIV type-2 (HIV-2) was implemented by June 1992. The risk for HIV transmission by transfusion of screened blood is minimal. Nearly all cases of transfusion-associated HIV transmission are now caused by blood donated during the infectious window period (i.e., when recently infected donors are infectious but have not yet developed detectable levels of HIV antibody). When whole-virus-lysate enzyme immunosorbent assays (EIAs) were used to screen blood donations from 1985 through 1990, the average length of the window period was 45 days (95% confidence interval {CI}=34- 55 days) (3). The average window period of the most sensitive contemporary recombinant protein-based EIA for HIV-1 and HIV-2 antibodies is now 20 days less (4), yielding an average infectious window period of 25 days (95% CI=9-41 days) (5). The increased sensitivity of contemporary HIV-antibody EIAs, improved donor interviewing about behaviors associated with risk for HIV infection, and deferral of donors who test positive for HIV, hepatitis, human T-cell leukemia virus type 1 (HTLV-I), or syphilis have considerably improved the safety of the U.S. blood supply. In 1993, only approximately six per 100,000 blood donations collected by the American Red Cross tested positive for HIV antibody (6). In addition, only an estimated one in 450,000 to one in 660,000 donations per year (i.e., 18-27 donations) were infectious for HIV but were not detected by current screening tests (5). During the acute period of infection, tests for p24 antigen can detect HIV infection earlier than antibody tests. P24 antigen, the core structural protein of HIV, is detectable 2-3 weeks after HIV infection during the initial burst of virus replication associated with high levels of viremia (7,8). During this time, the blood of infected persons is highly infectious, and tests for p24 antigen are usually positive (9-11). On average, p24 antigen is detected an estimated 6 days before antibody tests become positive (4,9). When antibodies to HIV become detectable, p24 antigen is often no longer detectable because of antigen-antibody complexing and viral clearance (9-11). In August 1995, the Food and Drug Administration (FDA) recommended that all blood and plasma donations be screened for p24 antigen, effective within 3 months of licensure of a test labeled for such use (12). FDA recommended p24 screening as an additional safety measure because a) recent studies indicated that p24 screening reduces the infectious window period (4), b) implementation of p24-antigen testing had become logistically feasible for mass screening, and c) such testing would reduce the risk for HIV infection for persons who receive donated blood or blood products. Among the 12 million annual blood donations in the United States, p24-antigen screening is expected to detect four to six infectious donations that would not be identified by other screening tests. If each of these units were divided into an average of 1.8 blood components (13), antigen testing would result in removal of an estimated seven to 11 infectious components each year that would otherwise be available for transfusion. FDA regards donor screening for p24 antigen as an interim measure pending the availability of technology that would further reduce the risk for HIV transmission from blood donated during the infectious window period. This report provides guidelines for a) interpreting p24-antigen-assay results, b) counseling and follow-up of blood and plasma donors who have positive or indeterminate antigen-test results, and c) using p24-antigen testing in settings other than blood banks. These guidelines may be modified when additional information concerning antigen testing under mass screening conditions is collected and analyzed. P24-ANTIGEN-TEST ALGORITHM AND INTERPRETATION OF TEST RESULTS The p24-antigen screening assay is an EIA performed on serum or plasma. If the first screening test is nonreactive, the test result is reported as negative (see Definitions used in interpreting HIV-1 p24-antigen tests according to FDA recommendations (Table_B1)). If the first screening test is reactive, the p24 EIA is repeated in duplicate. If both duplicate tests are nonreactive, the test result is reported as negative. If at least one of the repeated p24 EIA tests is reactive, the test is considered repeatedly reactive; the donation is then discarded, the donor is deferred from donating blood, and a more specific assay (the neutralization assay) is performed to verify the presence of p24 antigen. The neutralization assay should be performed before informing donors of test results. As specified by FDA (12), donations collected within 3 months of a repeatedly reactive p24-antigen test (regardless of neutralization-assay results) should be quarantined pending results of repeat donor testing for antigen and antibody to HIV. Sample storage requirements and time restrictions specified in the test kit package insert should be closely followed to prevent sample deterioration, and thus, invalid test results. FDA recommends that units of whole blood, blood components, source leukocytes, and source plasma obtained from donors whose blood samples are repeatedly reactive on p24-antigen screening tests be destroyed or quarantined and not used for transfusion or for manufacturing into injectable products. Available data indicate that the p24-antigen assay is sensitive and specific. The specificity of the p24-antigen test was calculated by two test-kit manufacturers to be 99.95% and 99.93% (Table_1) (14). Additionally, in one study of 514,000 donations, 225 were repeatedly reactive on the screening test. Of these donations, neutralization tests were negative for 220 (98%). Five (2%) donations were negative on neutralization tests and had detectable HIV antibodies. Testing by the polymerase chain reaction (PCR) for HIV DNA and RNA was performed on 120 of these non-neutralizing blood donations, all of which were negative for HIV. Follow-up samples were obtained from 79 of these donors, all of which were negative for HIV-1 antibody (15). In a prospective study conducted from September 1993 through September 1995, a total of 305,989 donations were tested for p24 antigen; three donors had both repeatedly reactive p24-antigen EIA screening-test results and positive neutralization results (two of whom were also HIV-antibody positive), and 223 donors had repeatedly reactive p24-antigen EIA screening-test results and negative neutralization results. Of those donors who had negative neutralization results, 81 later returned to donate blood again. Sixty-five of these donors had negative test results for HIV-1/HIV-2 antibody and for antigen EIA and neutralization. However, 16 donors who were HIV-1/HIV-2 antibody negative on subsequent donations continued to have repeatedly reactive p24-antigen EIA screening tests that did not neutralize (16). A recent study of 51 seroconversion panels has yielded an estimate of clinical sensitivity of the p24-antigen screening test in detecting blood donated during the infectious window period. An analysis of 69 preseroconversion samples that were positive for HIV-1 RNA by PCR demonstrated that the antigen test was reactive for 51 (74%) of those samples (M.P. Busch, personal communication, 1995). To assess sensitivity of the neutralization test, two antigen-test-kit manufacturers also performed neutralization testing on samples of blood from persons seroconverting to HIV. One manufacturer tested 102 repeatedly reactive specimens from 30 seroconverting plasma donors; 100% were positive on the neutralization assay. Similarly, a second manufacturer found that all 52 repeatedly reactive specimens from 25 seroconverting plasma donors were positive on the neutralization test (14). DONOR COUNSELING, FOLLOW-UP, AND DEFERRAL Counseling blood and plasma donors who have positive or indeterminate HIV-test results is an essential adjunct to HIV testing. Counseling in the blood-bank setting a) provides information about follow-up diagnostic evaluation and available medical, preventive, and psychosocial services and b) assists infected persons in preventing transmission to others. HIV counseling should be conducted in accordance with PHS standards and guidelines (17,18). PHS guidelines for notification and counseling of donors who have repeatedly reactive antigen-test results are based on available data. These guidelines may be modified after the collection and analysis of additional information concerning antigen testing under mass screening conditions. Donors Who Have Positive P24-Antigen-Test Results Donors whose HIV-antibody-test results are negative, but whose screening-test results for HIV antigen are repeatedly reactive and neutralization-assay results are positive, should be counseled that they are probably infected with HIV (Figure_1) (Figure_1C). Donors who have such test results should be notified promptly after a positive neutralization test. Prompt notification is important because persons who are newly infected with HIV and do not have HIV antibodies often have high viral titers and may be at high risk for transmitting HIV infection (7). According to FDA recommendations, donors who have repeatedly reactive and neutralizing p24-antigen tests should be advised that they are permanently deferred from future blood and plasma donation. Donors who have repeatedly reactive EIA and neutralizing HIV antigen tests should have their results confirmed by follow-up antibody testing; diagnosis of HIV infection should not be made on the basis of p24-antigen-test results alone. Arrangements for follow-up antibody testing should be incorporated into routine counseling. Because the time between detection of antigen and antibody is estimated to be an average of 6 days, donors who have positive p24-antigen-test results can be offered repeat antigen and antibody testing at any follow-up visit. If repeat antigen tests remain reactive and antibody tests remain negative, antibody testing should be repeated after a minimum of 8 weeks to allow time for antibodies to develop. Pending repeat testing to confirm the initial positive antigen-test result, strategies to reduce transmission should be implemented immediately by the donor (e.g., abstaining from sexual intercourse and using condoms consistently and correctly). If follow-up antibody tests are positive--thus confirming HIV infection--infected persons should be referred for medical care; sex and needle-sharing partners of such persons should be advised to seek HIV testing at clinical sites. Some donors repeatedly have positive p24-antigen and negative HIV-antibody-test results, although such an occurrence is unusual. If after 8 weeks such persons still have negative antibody-test results, they should be referred for further medical evaluation, including determining CD4+ T-lymphocyte cell count or percentage. Testing for HIV by PCR or culture also may be helpful in determining HIV status; however, neither test is licensed for diagnosis of HIV infection. Donors Who Have Repeatedly Reactive P24-Antigen Screening-Test Results but Negative or Invalid Neutralization-Test Results In the blood- and plasma-donor population, which has a low prevalence of HIV infection, most repeatedly reactive p24-antigen screening-test results are expected to be false-positive reactions. Donors who have repeatedly reactive p24-antigen screening tests but have negative neutralization and HIV-antibody tests are probably not infected with HIV. Donors who have negative neutralization results should be counseled that their antigen screening tests were reactive but that their supplementary tests were negative, which likely represents a false-positive test. Although donors who have negative neutralization results should be reassured that their test result probably does not represent infection, they should be counseled to take HIV risk-reduction precautions until repeat testing has confirmed their HIV-infection status. An invalid neutralization result occurs when a sample is repeatedly reactive in the initial screening test, but in the neutralization assay, the neutralized sample and unneutralized control both fall below the cutoff level. Most frequently, invalid results occur when a screening-test value is low or borderline reactive in an uninfected person; however, invalid results can also occur when a screening-test value is low or borderline reactive in an infected person (15). An invalid result may also be the result of sample deterioration or antigen-antibody complex formation during storage. Donors who have invalid neutralization results should be counseled that their antigen screening tests were reactive but their supplementary tests were inconclusive because the neutralization-test results were invalid. These donors are probably not infected with HIV, but infection cannot be excluded. Donors who have invalid neutralization-test results should be counseled to take HIV risk-reduction precautions until their HIV-infection status is confirmed. Retesting a fresh sample may clarify the result. To exclude HIV infection, donors who have repeatedly reactive p24-antigen screening-test results, invalid or negative neutralization-test results, and negative HIV-antibody-test results can be offered repeat antigen and antibody testing at any follow-up visit. Negative antigen- and antibody-test results at the time of follow-up indicate the donor was not infected with HIV at the time of the initial test. For donor reentry purposes, or for diagnostic purposes among donors who continue to have reactive antigen screening-test results and negative antibody-test results, repeat antigen and antibody testing should be performed a minimum of 8 weeks after the initial repeatedly reactive antigen screening test. Negative antigen and antibody tests performed greater than or equal to 8 weeks after the initial repeatedly reactive antigen screening tests indicate the donor was not infected with HIV at the time of the initial test. If the donor's screening antigen test remains repeatedly reactive, neutralization test remains negative, and HIV-antibody tests are negative or indeterminate, the donor is probably not infected with HIV. Further follow-up and additional tests (i.e., CD4+ T-lymphocyte cell count or percentage, HIV PCR, and culture) may clarify infection status, although these tests are not licensed for diagnosis of HIV infection. FDA recommendations indicate that donors whose blood samples are repeatedly reactive by the p24 screening test and negative or invalid on the neutralization test should be temporarily deferred from donating blood or plasma for a minimum of 8 weeks. These persons should be counseled that donor deferral does not indicate infection, because the screening test on which the deferral was based was most likely a false-positive result. If, after 8 weeks, samples from such donors test negative by screening tests for p24 antigen and antibodies to HIV, the donors are not infected and can be reinstated as blood or plasma donors. However, if, after 8 weeks, samples test repeatedly reactive on the screening test for HIV antigen or are positive for antibody, the donors should be permanently deferred from donating blood or plasma--regardless of HIV-antigen neutralization-test results. IMPLICATIONS FOR OTHER HIV TEST SITES Initiation of p24-antigen screening of the blood supply may motivate some persons who are at high risk for HIV infection to donate blood to determine HIV status. Such an unintended inducement could offset the benefits of p24-antigen screening (19). Since 1983, PHS has discouraged persons at high risk for HIV infection from donating blood (20). Such persons should be discouraged from donating blood and plasma and encouraged to be tested for HIV antibody at other sites. Initiation of routine testing for p24 antigen in publicly funded HIV counseling and testing sites, physicians' offices, or other nonblood-bank settings is not recommended. Few additional infected persons would be identified by routine antigen testing in such settings, because the estimated average time from detection of p24 antigen to detection of antibody is only 6 days. In addition, recently infected persons may not have detectable levels of p24 antigen. However, antigen testing may be appropriate in certain circumstances, such as diagnosis of perinatally exposed children (21,22). Diagnostic testing for HIV infection in children greater than or equal to 18 months of age and adults should routinely consist of an HIV-1-antibody screening test and Western blot or immunofluorescent assay for confirmation of antibodies to HIV-1. CONCLUSIONS PHS is committed to maintaining a safe blood supply. To further promote this goal, FDA has recommended that all blood and plasma donations be screened with tests for HIV-1 p24 antigen because these test results frequently become positive before assays for HIV antibodies. Screening blood donors for p24 antigen is expected to remove four to six infectious donations from the blood supply each year that would not be removed by other screening tests. After implementation of p24-antigen screening, CDC will collaborate with blood- and plasma-collection agencies to evaluate the sensitivity, specificity, and positive predictive value of the p24-antigen test. As a result, the p24-antigen testing algorithm and counseling guidelines may be modified after additional data are collected and analyzed. References
Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Specificity of HIV-1 p24 antigen for use in screening blood and plasma donors (14).
=============================================================================================
Repeatedly
Study No. of Initially Repeatedly reactive and
Manufacturer population specimens reactive (%) reactive (%) neutralized (% *)
---------------------------------------------------------------------------------------------
A Blood donors 301,699 1,348 (0.45) 144 (0.05) 3 + ( 2.10)
B Blood and 10,270 47 (0.46) 8 (0.08) 1 & (12.50)
plasma donors
---------------------------------------------------------------------------------------------
* Percentage of specimens that were repeatedly reactive.
+ Two of three specimens were also antibody positive. The third specimen was from a donor
who subsequently showed no other evidence of HIV infection. The specificity of Manufacturer
A's antigen assay is approximately 99.95% (301,555 per 301,697) based on a) an assumed zero
prevalence of HIV-1 p24 antigen in random blood donors who have no other evidence of HIV-1
infection and b) additional testing using the neutralization test and Western blot analysis
for HIV-1 antibodies.
& The repeatedly reactive specimen was confirmed as being HIV-1 p24-antigen positive; the
donor subsequently seroconverted to antibodies to HIV-1. When the HIV-1
p24-antigen-positive donor was removed from the calculation, the specificity of
Manufacturer B's test was 99.93% (10,262 per 10,269; 95% confidence
interval=99.86%-99.97%).
=============================================================================================
Return to top. Table_B1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
=======================================================================
Definitions used in interpreting HIV-1 p24-antigen tests according
to FDA recommendations
-----------------------------------------------------------------------
Initially reactive Initial p24 EIA test is reactive.
Repeatedly reactive One or both duplicate p24 EIA retests is
(are) reactive.
Negative Initial p24 EIA test is not reactive; or,
initial p24 EIA test is reactive and both
duplicate p24 EIA retests are not reactive.
Positive P24 EIA test is repeatedly reactive and the
neutralization test is positive (i.e,
neutralizing).
Indeterminate P24 EIA test is repeatedly reactive and the
neutralization test is either negative
(i.e., non-neutralizing) or invalid.
=======================================================================
Return to top. Figure_1 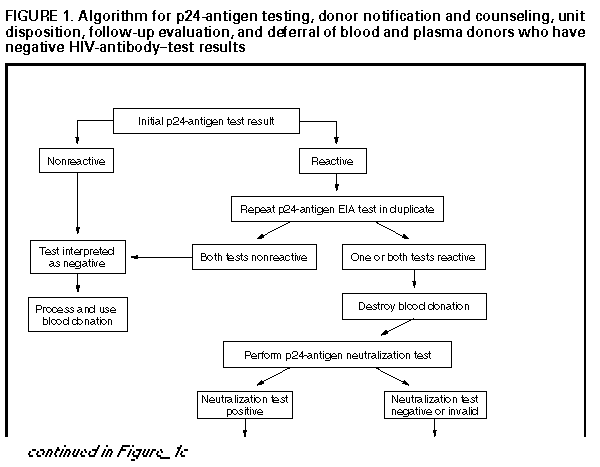 Return to top. Figure_1C 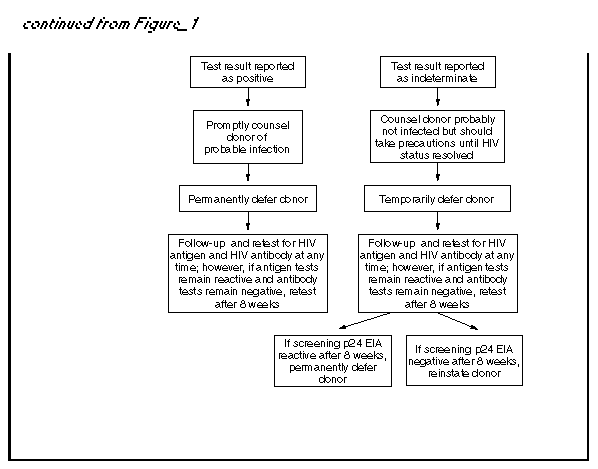 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|