 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Screening for Colorectal Cancer -- United States, 1992-1993, and New GuidelinesColorectal cancer is the third most commonly diagnosed cancer for both men and women in the United States and is the second leading cause of cancer-related deaths (1). During 1996, approximately 133,500 new cases of colorectal cancer will be diagnosed, and 54,900 persons will die from the disease (1). Recent evidence of the efficacy of colorectal cancer screening to reduce mortality was reviewed by the U.S. Preventive Services Task Force (USPSTF), an independent expert advisory panel to the Public Health Service (2). The revised USPSTF recommendations on cancer screening suggest that the risk for colorectal cancer-related mortality can be reduced by the use of specific screening tests (i.e., annual fecal occult blood testing {FOBT} and/or periodic flexible sigmoidoscopy for persons aged greater than or equal to 50 years) *. To estimate the prevalence of colorectal cancer screening practices, CDC analyzed data on use of colorectal cancer screening methods from the 1992 and 1993 Behavioral Risk Factor Surveillance System (BRFSS). This report summarizes the results of that analysis, which documents low rates of use of colorectal cancer screening and underscores the need for efforts to increase screening. In 1993, a total of 49 states and the District of Columbia participated in the BRFSS, a population-based, random-digit-dialed telephone survey of the U.S. civilian, noninstitutionalized population. A total of 38,063 respondents aged greater than or equal to 50 years were asked whether they ever had had a digital rectal examination (DRE) or a proctoscopic examination and when the last examination was performed. Data were weighted and aggregated, and composite estimates and standard errors were calculated using SUDAAN. Data are presented for the proportion of respondents reporting a DRE during the year preceding the interview and the proportion reporting proctosigmoidoscopy during the 5 years preceding the interview for selected groups (i.e., sex, race, age, annual household income, and education level). Race-specific data are presented because screening rates and death rates previously have varied by these categories; data are presented only for whites and blacks because numbers for other racial groups were too small to calculate precise estimates. Overall, 43% of respondents reported a DRE during the preceding year, and 28% reported a proctosigmoidoscopy during the preceding 5 years. Men were more likely than women to have had a proctosigmoidoscopy (33% and 24%, respectively) and to have had a DRE (47% and 40%, respectively) ((Figure_1) and (Figure_2)). Whites were more likely than blacks to have had a proctosigmoidoscopy (29% and 26%, respectively) and to have had a DRE (44% and 39%, respectively). The proportion of respondents reporting proctosigmoidoscopy increased with age, from 23% of persons aged 50-59 years to 32% of persons aged greater than or equal to 70 years. For both DRE and proctosigmoidoscopy, the proportion of respondents tested was directly related to income and level of education. Among those earning less than $15,000 annually, 35% reported a DRE, and 24% reported a proctosigmoidoscopy; among those earning greater than $50,000 annually, 55% reported a DRE, and 35% reported a proctosigmoidoscopy. Among those with less than 12 years of education, 37% reported a DRE, and 24% reported a proctosigmoidoscopy; among those with a college education, 49% reported a DRE, and 32% reported a proctosigmoidoscopy. Although there were differences in race-specific crude rates, these rates were similar when analyzed by education and income categories. In 1992, four states (California, Delaware, New Jersey, and New York) used a BRFSS module that included questions about FOBT. In these states, the overall proportion of persons reporting having had an FOBT during the year preceding the interview was 34% for men and 29% for women. Reported by: State Behavioral Risk Factor Surveillance System coordinators. Office of Disease Prevention and Health Promotion, US Dept of Health and Human Svcs. Epidemiology and Statistics Br, Div of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, CDC. Editorial NoteEditorial Note: Well-established risk factors for colorectal cancer include older age, male sex, inflammatory bowel disease, certain hereditary conditions (e.g., familial polyposis), and family history of colorectal cancer. In addition, dietary fat, alcohol, sedentary lifestyle, and obesity are potential risk factors (3). Because the well-established risk factors are not amenable to change, the primary strategy for preventing colorectal cancer deaths is to detect and remove precancerous polyps or to detect and treat cancer in its earliest stages. The efficacy of colorectal cancer screening by FOBT and sigmoidoscopy as means for reducing colorectal cancer deaths has been well documented (4-6). The findings in this report document low overall rates of use of colorectal cancer screening in the United States. DRE was the most commonly used test for colorectal cancer, probably reflecting its practical incorporation into routine physical examinations. However, DRE can detect tumors only in the distal 10 cm of the colon, and the efficacy of DRE has not been documented. Although FOBT data were available only from four states, because the use of DRE and proctosigmoidoscopy in these states was not substantially different from the nationwide average, the FOBT data may be representative of the national average. The BRFSS questionnaire does not distinguish between tests conducted for diagnosis and for screening. However, because proctosigmoidoscopies are more likely to be used for diagnosis than FOBT and DRE (41% versus 24% and 20%, respectively) (7), proctosigmoidoscopy may be the least used screening test for colorectal cancer. The findings in this report are subject to at least two limitations. First, because the BRFSS is a telephone survey, persons without telephones are not represented. Therefore, because of the association between absence of residential telephones and lower socioeconomic status, persons without telephones may be less likely to have been screened and testing rates may have been overestimated. Second, the BRFSS findings are based on self-reports and have not been validated. The findings in this report document the need for efforts to increase screening for colorectal cancer, especially by using methods shown to be effective (e.g., FOBT and proctosigmoidoscopy). However, evidence is insufficient to determine which of these screening methods is preferable or whether the combination of FOBT and sigmoidoscopy produces greater benefits than either test alone. The prevalences of screening for colorectal cancer are lower than those for screening for breast and cervical cancer; the substantial increase during the 1980s in the use of mammography has not occurred for use of colorectal cancer screening tests (8-10). Public health officials and policy makers should intensify efforts to educate providers and the public about the effectiveness of screening, to promote widespread use of the colorectal cancer screening guidelines developed by USPSTF, and to ensure access to screening tests for persons with low income. References
Figure_1 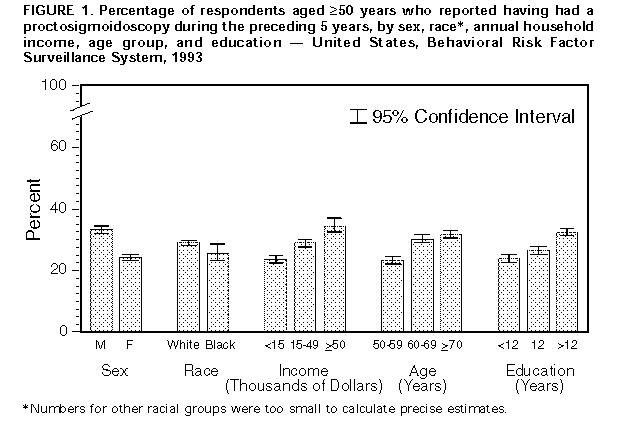 Return to top. Figure_2 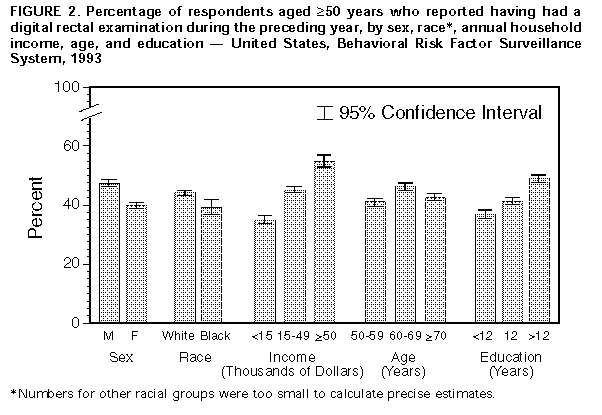 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|