 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. APPENDIX A Microscopic Procedures for Diagnosing MalariaTo establish the diagnosis of malaria, a blood smear must be prepared from fresh finger-prick blood (Figure_A1 and Figure_A2). * The thin smear is fixed in methanol before staining; the thick smear is stained unfixed. Many hospitals have a Wright-Giemsa stain available, which is acceptable; however, Wright stain alone will not reliably stain Plasmodium parasites. For best results, the smear should be stained with a 3% Giemsa solution (pH of 7.2) for 30-45 minutes. In P. falciparum infections, the parasite density should be estimated by counting the percentage of red blood cells infected_not the number of parasites_under an oil immersion on a thin film. Thick blood smears are more sensitive in detecting malaria parasites because the blood is concentrated, allowing a greater volume of blood to be examined. However, thick smears are more difficult to read, and thin smears may be preferred by laboratories that have limited experience. Plasmodium parasites are always intracellular, and they demonstrate, if stained correctly, blue cytoplasm with a red chromatin dot. Common errors in reading malaria smears are caused by platelets overlying a red blood cell, concern about missing a positive slide, and misreading artifacts as parasites. Persons suspected of having malaria but whose blood smears do not demonstrate the presence of parasites should have blood smears repeated approximately every 12-24 hours for 3 consecutive days. If smears remain negative, then the diagnosis of malaria is unlikely. For rapid diagnosis, make the thick and thin films on separate slides. Air dry the thin film, fix it with methyl alcohol, and immediately stain it. If no parasites are found on the thin film, wait until the thick film is dry and examine it for organisms that may not have been detected on the thin preparation.
Figure_A1 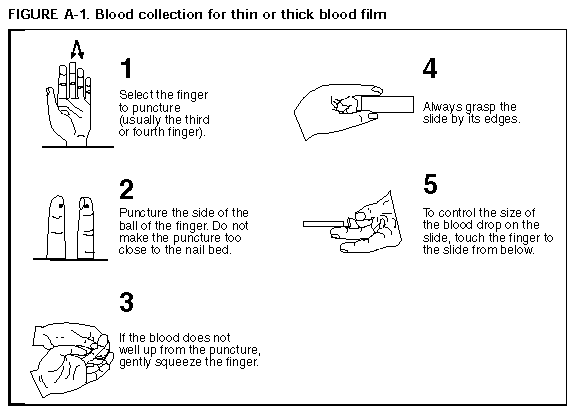 Return to top. Figure_A2 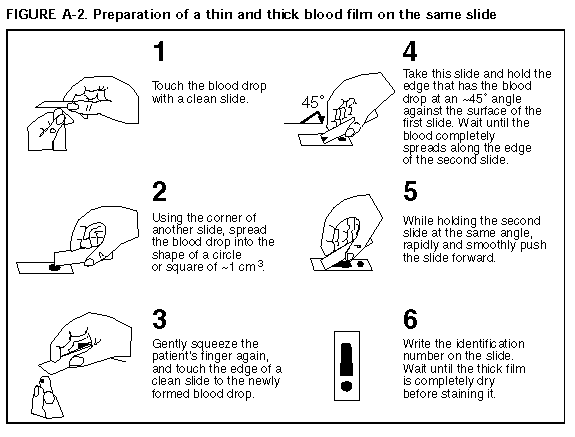 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|