 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Elimination of Haemophilus influenzae Type b Disease Among Infants and Children -- United States, 1993-1994Before effective vaccines were available, Haemophilus influenzae type b (Hib) was the most common cause of bacterial meningitis among children in the United States. Since the introduction of Hib conjugate vaccines in 1988, the incidence of invasive Hib infection has declined by at least 95% among infants and children (1,2). As part of the Childhood Immunization Initiative (CII), the Public Health Service has included Hib disease among children aged less than 5 years as one of the vaccine-preventable diseases targeted for elimination in the United States by 1996 (3). This report summarizes provisional data about invasive Hi disease during 1993-1994 based on information from three surveillance systems: the National Notifiable Diseases Surveillance System (NNDSS), the National Bacterial Meningitis and Bacteremia Reporting System (NBMBRS), and a multistate laboratory-based surveillance system. National Surveillance State health agencies reported weekly provisional notifiable disease data to NNDSS through the National Electronic Telecommunications System for Surveillance (NETSS) (4,5). Because the primary purpose of NNDSS is timely nationwide surveillance, the information transmitted included only basic demographic data about persons with invasive Hi disease. The capacity for the electronic transmission of critical supplemental information (e.g., the type of clinical illness, serotype causing disease, Hib vaccination status, and clinical outcome) for cases of Hi disease is available through NETSS and is used consistently by approximately half of the states. NBMBRS is a collaborative effort initiated in 1977 by CDC, state health departments, and the Council of State and Territorial Epidemiologists to collect information about invasive bacterial diseases in the United States. NBMBRS includes detailed information about each case identical to the supplemental information transmitted through NETSS. Approximately 20 states participate consistently in reporting through the NBMBRS. From 1993 to 1994, the incidence of invasive Hi disease among children aged less than 5 years reported to the NNDSS decreased 29% (from 2.4 cases per 100,000 to 1.7 cases per 100,000, respectively), a trend similar to that reported for 1992-1993 Figure_1 (2). However, the total number of cases among children aged less than 5 years reported during the first 4 months of 1995 (105) is similar to that during the same period in 1994 (104). Supplemental case information was reported to CDC by 35 states and was obtained on request from the remaining states. Of the 340 cases of invasive Hi disease among children aged less than 5 years reported in 1994, supplemental information was available for 259 (76%). Of these, serotype data were available for 139 (54%) -- 41% of all reported cases. Hib accounted for 82 (59%) of the isolates for which serotype was known. Of the 60 (73%) cases of Hib disease for which information on age and vaccination status was available, none of the 12 children aged greater than 15 months had received four doses of Hib vaccine Table_1. Two of the 19 children aged 7-15 months had received three vaccine doses, while most (17) had not completed the recommended primary series. Nearly half (29) were aged less than or equal to 6 months, below the age recommended for completion of the full three-dose primary series of the most commonly used Hib vaccines; of these, five had received two doses of vaccine. Laboratory-Based Surveillance The laboratory-based system coordinated by CDC includes surveillance projects with a total population of 10.4 million persons in four areas (three counties in the San Francisco Bay area, eight counties in metropolitan Atlanta, four counties in Tennessee, and the state of Oklahoma). Information routinely obtained for all cases of invasive Hi disease included serotype, clinical syndrome, outcome, vaccination status, and demographic information. Because blacks were overrepresented in the surveillance population, rates were race-adjusted to the 1990 age-specific U.S. population. The incidence of Hib disease among children aged less than 5 years declined from 1989 to 1993 but was stable from 1993 to 1994 (1.5 and 1.4 cases per 100,000, respectively) Figure_2. Information about vaccination status was available for eight of the 10 children aged less than 5 years with invasive Hib disease reported in 1994. None of the infants had received two or more doses of vaccine, although three were aged 8 months and should have received three doses. The two children for whom vaccination information was not available were aged greater than 16 months. Based on a projection of these age-specific and race-adjusted incidence rates, an estimated 280 cases of Hib disease occurred among children aged less than 5 years in 1994 compared with an estimated 290 cases in 1993. During 1993 and 1994, Hib accounted for 37% of all the Hi isolates obtained from children aged less than 5 years. Reported by: G Rothbrock, Bur of Disease Control, Oakland, California. L Smithee, MS, Oklahoma State Dept of Health. M Rados, MS, Dept of Preventive Medicine, Vanderbilt Medical Center, Nashville, Tennessee. W Baughman, MSPH, Veterans' Administration Medical Svcs, Atlanta. National Immunization Program; National Center for Infectious Diseases; Epidemiology Program Office, CDC. Editorial NoteEditorial Note: The goal to eliminate Hib disease among children aged less than 5 years is feasible because of the availability of Hib conjugate vaccines that are efficacious in children and reduce carriage of the organism, thereby interrupting transmission of infection. During 1988-1992, the incidence of invasive Hib disease declined rapidly among children; however, the findings in this report indicate that, since 1992, the rate of decline among children has slowed. This report also underscores two barriers to the elimination of invasive Hib disease among children: 1) the absence of accurate national surveillance for Hib incidence because of the lack of serotype information for most invasive Hi disease cases among children, and 2) the continued occurrence of disease among undervaccinated children and among infants too young to have completed the primary series of Hib vaccination. Serotype information for cases of invasive Hi disease is essential to evaluate the changing epidemiology of Hib disease during a period of low disease incidence. Surveillance data indicate that a decreasing proportion of Hi cases are caused by Hib -- which in the past was responsible for greater than 90% of all Hi disease. Thus, the decline in the incidence of Hi disease among children observed in NNDSS data for 1994 may not have resulted from a reduction in Hib disease; data from laboratory-based surveillance suggests that, during 1993-1994, incidence of Hib disease remained stable. Because serotype information could be obtained for only 41% of cases reported to the NNDSS in 1994, the true incidence of Hib disease among children in the United States cannot be estimated from these data. In the national surveillance data, the higher proportion of Hib among Hi isolates of known serotype probably reflects incomplete serotyping information and preferential reporting of Hib cases in the national data. Both national and laboratory-based surveillance findings indicate that Hi disease now occurs primarily among undervaccinated children and among infants too young to have completed the primary series of vaccination. However, based on the findings from CDC's National Health Interview Survey, the quarterly levels of coverage with three or more doses of Hib vaccine among children aged 19-35 months increased significantly from the third quarter of 1993 (60%) to the second quarter of 1994 (76%) (6). Although overall Hib vaccination coverage may be increasing, population groups with low levels of vaccination coverage probably contribute to the ongoing occurrence of disease (7). The findings in this report indicate that no cases of vaccine failure were identified through laboratory-based surveillance in a population of 10.5 million. The small proportion of Hib cases reported through national surveillance among children who had received at least three doses of Hib vaccine suggests vaccine failure occurs infrequently, but is still consistent with previous reports showing extremely high efficacy of current vaccines (8-10). As a larger proportion of Hib cases is detected and investigated, more complete evaluations of cases among fully vaccinated persons will be possible. To meet the 1996 CII objectives to eliminate invasive Hib disease among children aged less than 5 years, CDC recommends two measures. First, national surveillance for Hi should be strengthened. To optimize surveillance efforts, case reports should satisfy four criteria: 1) because Hib vaccines protect against Hi serotype b organisms only, serotyping should be obtained for all cases of invasive Hi disease -- state health departments are encouraged to identify laboratories to ensure that serotyping is available for all Hi isolates; 2) to improve characterization of groups at risk for undervaccination and Hib disease, vaccination status of all children with invasive Hib disease should be assessed; 3) to ensure continued high levels of vaccine effectiveness and to enable systematic evaluation of factors associated with vaccine failure in persons with Hib disease, the date, vaccine manufacturer, and lot number for each Hib vaccination should be reported; and 4) important indicators of the severity of Hi infections should be reported, including the type of clinical syndrome, specimen source (e.g., cerebrospinal fluid, blood, or joint fluid), and clinical outcome. Second, timely vaccination and vaccine coverage should be increased. Because conjugate vaccines reduce Hib carriage and interrupt transmission of the organism, timely vaccination of all children also should eliminate disease among infants who are too young to be completely vaccinated. References
Figure_1 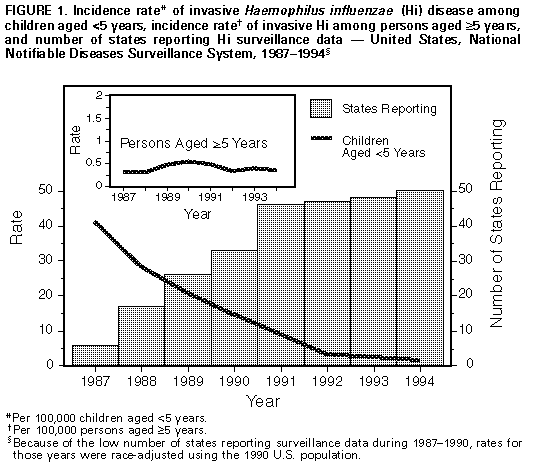 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Number of children aged <5 years with invasive Haemophilus
influenzae type B (Hib) disease, by age group and number of Hib
vaccine doses received -- United States, 1994 *
==========================================================================
No. vaccine doses +
Age group ----------------------------------------------
(mos) 0 1 2 3 Total
--------------------------------------------------------------------------
0- 3 9 8 0 0 17
4- 6 1 6 5 0 12
7-15 6 5 6 2 19
16-59 7 1 0 4 & 12
Total 23 20 11 6 60
--------------------------------------------------------------------------
* Reported through the National Notifiable Diseases Surveillance System
and the National Bacterial Meningitis and Bacteremia Reporting
System.
+ Doses administered within 10 days of onset of illness were not
included.
& These children were aged 2 years (two), 3 years (one), and 4 years
(one).
==========================================================================
Return to top. Figure_2 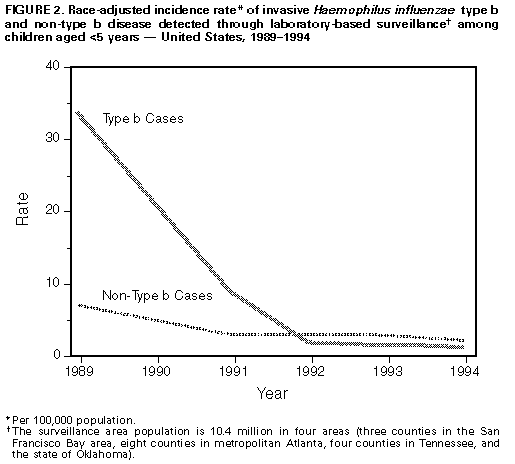 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|