 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Recommended Adult Immunization Schedule --- United States, October 2005--September 2006The Advisory Committee on Immunization Practices (ACIP) annually reviews the recommended Adult Immunization Schedule to ensure that the schedule reflects current recommendations for the use of licensed vaccines. In June 2005, ACIP approved the Adult Immunization Schedule for October 2005--September 2006. This schedule has also been approved by the American Academy of Family Physicians and the American College of Obstetricians and Gynecologists. Changes in the Schedule for October 2005--September 2006The 2005--2006 schedule differs from the previous schedule as follows:
The Adult Immunization Schedule is available in English and Spanish at http://www.cdc.gov/nip/recs/adult-schedule.htm. General information about adult immunization, including recommendations concerning vaccination of persons with HIV and other immunosuppressive conditions, is available from state and local health departments and from the National Immunization Program at http://www.cdc.gov/nip. Vaccine information statements are available at http://www.cdc.gov/nip/publications/vis. ACIP statements for each recommended vaccine can be viewed, downloaded, and printed from the National Immunization Program website at http://www.cdc.gov/nip/publications/acip-list.htm. Instructions for reporting adverse events to the Vaccine Adverse Event Reporting System are available at http://www.vaers.hhs.gov or by telephone, 800-822-7967. References
Figure 1 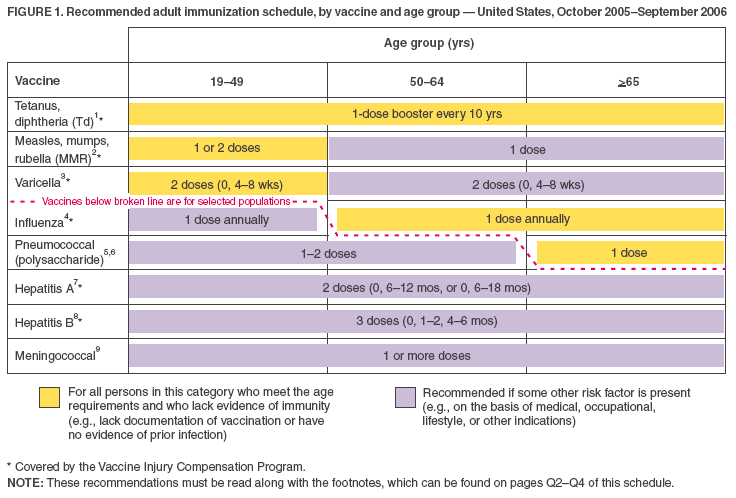 Return to top. Figure 2 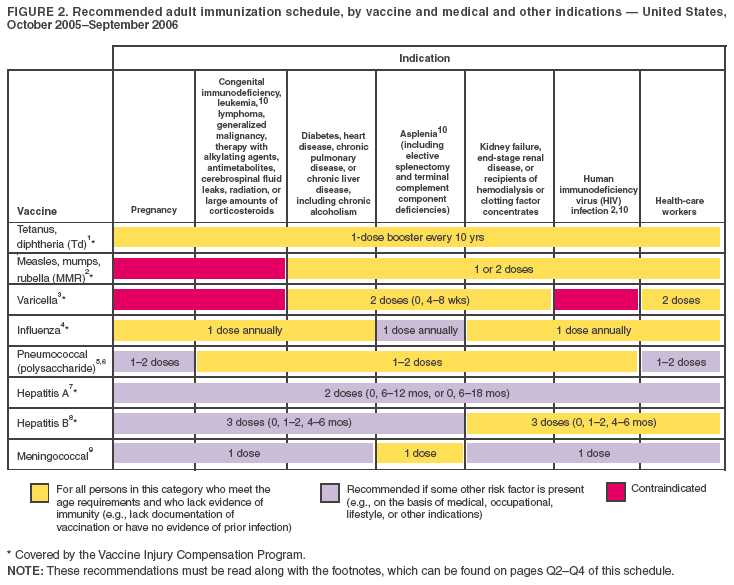 Return to top. Approved by the Advisory Committee on Immunization Practices, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians 1. Tetanus and diphtheria (Td) vaccination. Adults with uncertain histories of a complete primary vaccination series with diphtheria and tetanus toxoid--containing vaccines should receive a primary series using combined Td toxoid. A primary series for adults is 3 doses; administer the first 2 doses at least 4 weeks apart and the third dose 6--12 months after the second. Administer 1 dose if the person received the primary series and if the last vaccination was received >10 years previously. Consult the ACIP statement for recommendations for administering Td as prophylaxis in wound management (http://www.cdc.gov/mmwr/preview/mmwrhtml/00041645.htm). The American College of Physicians Task Force on Adult Immunization supports a second option for Td use in adults: a single Td booster at age 50 years for persons who have completed the full pediatric series, including the teenage/young adult booster. A newly licensed tetanus-diphtheria-acellular--pertussis vaccine is available for adults. ACIP recommendations for its use will be published. 2. Measles, mumps, rubella (MMR) vaccination. Measles component: adults born before 1957 can be considered immune to measles. Adults born during or after 1957 should receive >1 dose of MMR unless they have a medical contraindication, documentation of >1 dose, history of measles based on health-care provider diagnosis, or laboratory evidence of immunity. A second dose of MMR is recommended for adults who 1) were recently exposed to measles or in an outbreak setting; 2) were previously vaccinated with killed measles vaccine; 3) were vaccinated with an unknown type of measles vaccine during 1963--1967; 4) are students in postsecondary educational institutions; 5) work in a health-care facility; or 6) plan to travel internationally. Withhold MMR or other measles-containing vaccines from HIV-infected persons with severe immunosuppression. Mumps component: 1 dose of MMR vaccine should be adequate for protection for those born during or after 1957 who lack a history of mumps based on health-care provider diagnosis or who lack laboratory evidence of immunity. Rubella component: administer 1 dose of MMR vaccine to women whose rubella vaccination history is unreliable or who lack laboratory evidence of immunity. For women of childbearing age, regardless of birth year, routinely determine rubella immunity and counsel women regarding congenital rubella syndrome. Do not vaccinate women who are pregnant or who might become pregnant within 4 weeks of receiving vaccine. Women who do not have evidence of immunity should receive MMR vaccine upon completion or termination of pregnancy and before discharge from the health-care facility. 3. Varicella vaccination. Varicella vaccination is recommended for all adults without evidence of immunity to varicella. Special consideration should be given to those who 1) have close contact with persons at high risk for severe disease (health-care workers and family contacts of immunocompromised persons) or 2) are at high risk for exposure or transmission (e.g., teachers of young children; child care employees; residents and staff members of institutional settings, including correctional institutions; college students; military personnel; adolescents and adults living in households with children; nonpregnant women of childbearing age; and international travelers). Evidence of immunity to varicella in adults includes any of the following: 1) documented age-appropriate varicella vaccination (i.e., receipt of 1 dose before age 13 years or receipt of 2 doses [administered at least 4 weeks apart] after age 13 years); 2) U.S.-born before 1966 or history of varicella disease before 1966 for non-U.S.--born persons; 3) history of varicella based on health-care provider diagnosis or parental or self-report of typical varicella disease for persons born during 1966--1997 (for a patient reporting a history of an atypical, mild case, health-care providers should seek either an epidemiologic link with a typical varicella case or evidence of laboratory confirmation, if it was performed at the time of acute disease); 4) history of herpes zoster based on health-care provider diagnosis; or 5) laboratory evidence of immunity. Do not vaccinate women who are pregnant or who might become pregnant within 4 weeks of receiving the vaccine. Assess pregnant women for evidence of varicella immunity. Women who do not have evidence of immunity should receive dose 1 of varicella vaccine upon completion or termination of pregnancy and before discharge from the health-care facility. Dose 2 should be administered 4--8 weeks after dose 1. 4. Influenza vaccination. Medical indications: chronic disorders of the cardiovascular or pulmonary systems, including asthma; chronic metabolic diseases, including diabetes mellitus, renal dysfunction, hemoglobinopathies, or immunosuppression (including immunosuppression caused by medications or HIV); any condition (e.g., cognitive dysfunction, spinal cord injury, seizure disorder, or other neuromuscular disorder) that compromises respiratory function or the handling of respiratory secretions or that can increase the risk for aspiration; and pregnancy during the influenza season. No data exist on the risk for severe or complicated influenza disease among persons with asplenia; however, influenza is a risk factor for secondary bacterial infections that can cause severe disease among persons with asplenia. Occupational indications: health-care workers and employees of long-term--care and assisted living facilities. Other indications: residents of nursing homes and other long-term--care and assisted living facilities; persons likely to transmit influenza to persons at high risk (i.e., in-home household contacts and caregivers of children aged 0--23 months, or persons of all ages with high-risk conditions), and anyone who wishes to be vaccinated. For healthy, nonpregnant persons aged 5--49 years without high-risk conditions who are not contacts of severely immunocompromised persons in special care units, intranasally administered influenza vaccine (FluMist®) may be administered in lieu of inactivated vaccine. 5. Pneumococcal polysaccharide vaccination. Medical indications: chronic disorders of the pulmonary system (excluding asthma); cardiovascular diseases; diabetes mellitus; chronic liver diseases, including liver disease as a result of alcohol abuse (e.g., cirrhosis); chronic renal failure or nephrotic syndrome; functional or anatomic asplenia (e.g., sickle cell disease or splenectomy [if elective splenectomy is planned, vaccinate at least 2 weeks before surgery]); immunosuppressive conditions (e.g., congenital immunodeficiency, HIV infection [vaccinate as close to diagnosis as possible when CD4 cell counts are highest], leukemia, lymphoma, multiple myeloma, Hodgkin disease, generalized malignancy, or organ or bone marrow transplantation); chemotherapy with alkylating agents, antimetabolites, or long-term systemic corticosteroids; and cochlear implants. Other indications: Alaska Natives and certain American Indian populations; residents of nursing homes and other long-term--care facilities. 6. Revaccination with pneumococcal polysaccharide vaccine. One-time revaccination after 5 years for persons with chronic renal failure or nephritic syndrome; functional or anatomic asplenia (e.g., sickle cell disease or splenectomy); immunosuppressive conditions (e.g., congenital immunodeficiency, HIV infection, leukemia, lymphoma, multiple myeloma, Hodgkin disease, generalized malignancy, or organ or bone marrow transplantation); or chemotherapy with alkylating agents, antimetabolites, or long-term systemic corticosteroids. For persons aged >65 years, one-time revaccination if they were vaccinated >5 years previously and were aged <65 years at the time of primary vaccination. 7. Hepatitis A vaccination. Medical indications: persons with clotting-factor disorders or chronic liver disease. Behavioral indications: men who have sex with men or users of illegal drugs. Occupational indications: Persons working with hepatitis A virus (HAV)--infected primates or with HAV in a research laboratory setting. Other indications: persons traveling to or working in countries that have high or intermediate endemicity of hepatitis A (for list of countries, see http://www.cdc.gov/travel/diseases.htm#hepa) as well as any person wishing to obtain immunity. Current vaccines should be administered in a 2-dose series at either 0 and 6--12 months, or 0 and 6--18 months. If the combined hepatitis A and hepatitis B vaccine is used, administer 3 doses at 0, 1, and 6 months. 8. Hepatitis B vaccination. Medical indications: hemodialysis patients (use special formulation [40 µg/mL] or two 20-µg/mL doses) or patients who receive clotting-factor concentrates. Occupational indications: health-care workers and public-safety workers who have exposure to blood in the workplace and persons in training in schools of medicine, dentistry, nursing, laboratory technology, and other allied health professions. Behavioral indications: injection-drug users; persons with more than one sex partner during the previous 6 months; persons with a recently acquired sexually transmitted disease (STD); and men who have sex with men. Other indications: household contacts and sex partners of persons with chronic hepatitis B virus (HBV) infection; clients and staff members of institutions for developmentally disabled persons; all clients of STD clinics; inmates of correctional facilities; and international travelers who will be in countries with high or intermediate prevalence of chronic HBV infection for more than 6 months (for list of countries, see http://www.cdc.gov/travel/diseases.htm#hepa). 9. Meningococcal vaccination. Medical indications: adults with anatomic or functional asplenia or terminal complement component deficiencies. Other indications: first-year college students living in dormitories; microbiologists who are routinely exposed to isolates of Neisseria meningitidis; military recruits; and persons who travel to or reside in countries in which meningococcal disease is hyperendemic or epidemic (e.g., the "meningitis belt" of sub-Saharan Africa during the dry season [December--June]), particularly if contact with local populations will be prolonged. Vaccination is required by the government of Saudi Arabia for all travelers to Mecca during the annual Hajj. Meningococcal conjugate vaccine is preferred for adults meeting any of the above indications who are aged <55 years, although meningococcal polysaccharide vaccine (MPSV4) is an acceptable alternative. Revaccination after 5 years might be indicated for adults previously vaccinated with MPSV4 who remain at high risk for infection (e.g., persons residing in areas in which disease is epidemic). 10. Selected conditions for which Haemophilus influenzae type b (Hib) vaccine may be used. Hib conjugate vaccines are licensed for children aged 6--71 months. No efficacy data are available on which to base a recommendation concerning use of Hib vaccine for older children and adults with the chronic conditions associated with an increased risk for Hib disease. However, studies suggest good immunogenicity in patients who have sickle cell disease, leukemia, or HIV infection or who have had splenectomies; administering vaccine to these patients is not contraindicated. The Recommended Adult Immunization Schedule has been approved by the Advisory Committee on Immunization Practices, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians. The standard MMWR footnote format has been modified for publication of this schedule. Suggested citation: Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule---United States, October 2005--September 2006. MMWR 2005;54:Q1--Q4.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 10/13/2005 |
|||||||||
|