 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Pertussis --- United States, 1997--2000Pertussis was a major cause of morbidity and mortality among infants and children in the United States during the prevaccine era (i.e., before the mid-1940s). Following the introduction and widespread use of whole-cell pertussis vaccine combined with diphtheria and tetanus toxoids (DTP) among infants and children in the late 1940s, the incidence of reported pertussis declined to a historic low of 1,010 cases in 1976 (Figure 1). However, since the early 1980s, reported pertussis incidence has increased cyclically with peaks occurring every 3--4 years (1). In 1996, less reactogenic acellular pertussis vaccines (DTaP) were licensed and recommended for routine use among infants (2). This report summarizes national surveillance data for pertussis during 1997--2000 and assesses the effectiveness of pertussis vaccination in the United States during this period. The findings indicate that pertussis incidence continues to increase in infants too young to receive 3 doses of pertussis-containing vaccine and in adolescents and adults. Prevention efforts should be directed at maintaining high vaccination rates and managing pertussis cases and outbreaks. State health departments report weekly to CDC the number of pertussis cases, including demographic information, through the National Electronic Transmittal System for Surveillance. More detailed information about persons with pertussis, including clinical characteristics and vaccination history, is reported to CDC through the Supplementary Pertussis Surveillance System. Probable and confirmed pertussis cases are reported. A clinical case is defined as an acute cough illness lasting >14 days in a person with at least one pertussis-associated symptom (i.e., paroxysmal cough, post-tussive vomiting, or inspiratory whoop) or >14 days of cough in a person in an outbreak setting. A confirmed case is defined as a cough illness of any duration in a person with isolation of Bordetella pertussis, or a case that meets the clinical case definition and is confirmed by polymerase chain reaction (PCR) or by epidemiologic linkage to a laboratory-confirmed case. A probable case meets the clinical case definition but is not laboratory confirmed or epidemiologically linked to a laboratory-confirmed case. Vaccination coverage data are obtained from the National Health Interview Survey (NHIS) and the National Immunization Survey (NIS). NHIS is an annual cross-sectional household survey of the U.S. civilian population that collects data on vaccination status of children aged <6 years (3). Vaccination status is based on vaccination records or, when no records are available, on parental recall. NIS is a national telephone survey of the noninstitutionalized civilian population that estimates vaccination coverage among U.S. children aged 19--35 months (4). Vaccination histories are verified by vaccine providers. The effectiveness of pertussis vaccine (VE) among children aged 7--18 months in 1998 and 1999 was calculated using the screening method (5). During this time, most children received DTaP rather than DTP. The formula VE=1 - [PCV/(1-PCV)][(1-PPV)/PPV] was used; PCV is the proportion of children vaccinated and PPV is the proportion of the population vaccinated. All confirmed and probable pertussis cases were included. Children were considered vaccinated if they had received >3 doses of pertussis-containing vaccine. Children who were partially vaccinated (e.g., received 1 or 2 doses of vaccine) were excluded from calculations of PCV and PPV. Surveillance data during 1998--1999 were used to determine PCV, and data from NHIS for 1998 were used to estimate PPV. Data from NIS were used to determine the percentage of DTP, DTaP, and pediatric diphtheria and tetanus toxoids (DT) administered to children aged 7--18 months during 1998--1999. PCV and PPV were corrected for estimated use of DT. During 1997--2000, a total of 29,134 pertussis cases were reported to NETSS (6,564 in 1997; 7,405 in 1998; 7,298 in 1999, and 7,867 in 2000), for a crude average annual incidence rate of 2.7 per 100,000 population. Among 29,048 persons with pertussis for whom age was known, 8,390 (29%) were aged <1 year, 3,359 (12%) were aged 1--4 years, 2,835 (10%) were aged 5--9 years, 8,529 (29%) were aged 10--19 years, and 5,935 (20%) were aged >20 years. Average annual incidence rates were highest among infants aged <1 year (55.5 cases per 100,000 population) and lower in children aged 1--4 years (5.5), children aged 5--9 years (3.6), persons aged 10--19 years (5.5), and persons aged >20 years (0.8). Data on race were available for 17,308 (75%) of 23,113 patients aged <20 years. Of these, 15,124 (88%) were white, 1,438 (8%) were black, 316 (2%) were Asian/Pacific Islander, and 359 (2%) were American Indian/Alaska Native. Data on ethnicity were available for 16,543 (72%) patients aged <20 years. Of these, 2,715 (16%) were Hispanic. In comparison, the national population estimates for persons aged <20 years in 1998 were 79% white, 16% black, 4% Asian/Pacific Islander, and 1% American Indian/Alaska Native. Nationally, for all races, 15% of persons aged <20 years were Hispanic. Among persons with pertussis aged <20 years, males and females were represented equally; however, 67% of patients aged >20 years were female. Supplementary clinical data for persons with pertussis with known age was available for 28,187 (97%) cases. The proportion of pertussis patients who were hospitalized or had complications of pertussis was highest among infants aged <6 months, and decreased with increasing age (Table 1). Among infants aged <6 months, 63% were hospitalized, 12% had radiographically confirmed pneumonia, and 1% had seizures. Among all age groups, 26 cases of encephalopathy and 62 pertussis-related deaths were reported. According to NHIS data from 1998, 73% of children aged 7--18 months were vaccinated with >3 doses of DTP, DTaP, or DT vaccines. Surveillance data for 1998 and 1999 indicated that 58% of patients aged 7--18 months were vaccinated with >3 doses of DTaP, DTP, or DT. Compared with no doses of pertussis-containing vaccine, the VE for children aged 7--18 months receiving 3 doses was 88% (95% confidence interval [CI]=79%--93%). The VE was 91% (95% CI=82%--95%) for hospitalized patients, and 86% (95% CI=75%--92%) for nonhospitalized patients. Data do not now allow for separate estimation of VE for DTaP and DTP. According to NIS, use of DTaP, DTP, and DT in children aged <18 months during 1998--1999 was 66.3%, 33.1%, and 0.3%, respectively. Reported by: L Zanardi, MD, FB Pascual, MPH, K Bisgard, DVM, T Murphy, MD, M Wharton, MD, Epidemiology and Surveillance Div; E Maurice, MS, Data Management Div, National Immunization Program, CDC. Editorial Note:The increase in reported pertussis first noted in the 1980s continued throughout the 1990s (1,6). Compared with surveillance data for 1994--1996, the pertussis incidence rate among adolescents and adults has increased, 62% and 60%, respectively (6). The rate increased 11% among infants. In comparison, the incidence rate decreased 8% among children aged 1--4 years and remained stable among children aged 5--9 years. These increases could reflect a change in reporting or a true increase in incidence. In 1995, criteria for reporting a pertussis case changed in two ways: PCR became a method of confirmation, and data collection began for pertussis cases epidemiologically linked to another pertussis case. These changes primarily affected the reporting among patients aged >10 years. Although underreporting of mild or atypical disease is common (1), increased recognition and diagnosis of pertussis among older age groups probably contributed to the large increase of reported cases among adolescents and adults (7). Conversely, an increase in pertussis among infants too young to receive 3 doses of pertussis-containing vaccine suggests a true increase in pertussis circulation (8). Infants have been a well-recognized high-risk group; changes in diagnosis or reporting patterns in this age group are unlikely. Despite recent changes in pertussis diagnostic methods, the proportion of culture-confirmed cases among infants has increased (8). The screening VE estimate of 88% reflects the effectiveness of the overall vaccination program that, according to NIS, used approximately two thirds DTaP and one third DTP in children aged 7--18 months. This estimate is similar to the VE of 77%--90% previously estimated using the screening method for whole cell vaccine during 1992--1994 (9) and to VEs observed in clinical trials for acellular pertussis vaccines (2). The incidence of pertussis among children aged 6 months--4 years has remained stable throughout the 1990s (6), suggesting that protection offered by vaccination has continued with the introduction of DTaP. Thus, the increase in reported pertussis cases is not related to low VE or the introduction of acellular pertussis vaccines. Despite the effectiveness of vaccination, pertussis continues to occur in the United States among all age groups. The burden of disease remains highest in infants, who also have the highest rates for complications and death. In addition to maintaining high vaccination rates among preschool-aged children, prevention efforts should be directed at treatment of pertussis cases to prevent further spread of disease, use of antimicrobial prophylaxis in contacts of pertussis cases, and minimizing infant exposures to children and adults with cough illnesses (10). Studies among older children, adolescents, and adults examining pertussis disease burden and transmission of disease to infants might guide future policy decisions on the use of acellular pertussis vaccines among persons aged >7 years. Acknowledgement This report is based on data contributed by state and local health departments.
References
Table 1 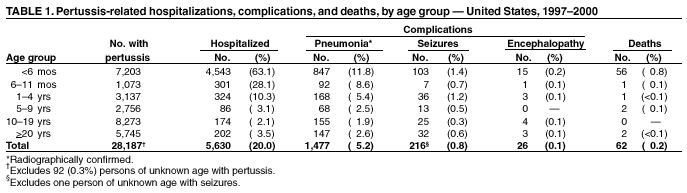 Return to top. Figure 1 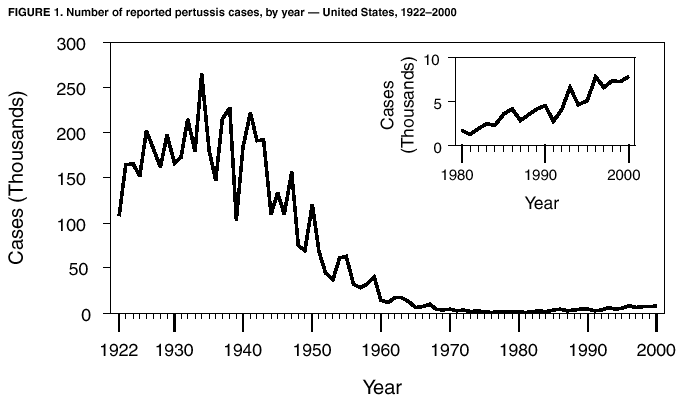 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/31/2002 |
|||||||||
This page last reviewed 1/31/2002
|