 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Childhood Influenza-Vaccination Coverage --- United States, 2002--03 Influenza SeasonChildren aged <2 years are at increased risk for influenza-related hospitalizations (1--3). Beginning in 2002, the Advisory Committee on Immunization Practices (ACIP) encouraged that, when feasible, all children aged 6--23 months, as well as household contacts and out-of-home caregivers for children aged <2 years, receive influenza vaccinations each influenza season (1). Beginning with the 2004--05 influenza season, ACIP strengthened the encouragement to a recommendation (4). Other children recommended to receive influenza vaccine continue to include those aged 6 months--18 years with certain high-risk medical conditions and those aged 6 months--18 years who are household contacts of persons at high risk for influenza complications (4). This report on childhood influenza-vaccination coverage for the 2002--03 influenza season provides a baseline for the continuing assessment of coverage among children aged 6--23 months. The findings demonstrate that, during the first year of the ACIP encouragement to vaccinate children aged 6--23 months against influenza, vaccination coverage was low, with substantial variability among states and urban areas. This report is based on data from the 2003 National Immunization Survey (NIS), an ongoing survey that provides estimates of vaccination coverage among noninstitutionalized children aged 19--35 months. Children included in the 2003 NIS were born during January 2000--July 2002. The survey is conducted in all 50 states and 28 selected urban areas (5). In 2003, entire influenza-vaccination histories were obtained for all children. Two measures of childhood influenza-vaccination coverage are reported: 1) receipt of one or more influenza vaccinations during September--December 2002 and 2) full vaccination (based on ACIP recommendations for 2 doses of influenza vaccine for previously unvaccinated children aged <9 years and 1 dose for previously vaccinated children aged <9 years) (4). Children were considered fully vaccinated if they had 1) received no doses of influenza vaccine before September 1, 2002, but then received 2 doses from September 1 through either the date of interview or January 31, 2003, or 2) received >1 dose of influenza vaccine before September 1 and then received >1 dose during September--December 2002. Because children aged <6 months are not eligible for vaccination and because the encouragement (and now the recommendation) calls for vaccination of children aged 6--23 months, analyses for both measures included only those children who were aged 6--23 months during the entire span of September--December 2002. In the 2003 NIS, the overall response rate for eligible households was 62.7%, and 13,831 children (unweighted) met the age criteria for this assessment. Of these, 7.4% (+0.7) received one or more influenza vaccinations, and 4.4% (+0.5) were fully vaccinated (Table). Substantial variability in influenza coverage was observed among states and selected urban areas. Percentages of children receiving one or more influenza vaccinations ranged from 2.2% (+2.1) in El Paso County, Texas, to 26.6% (+8.0) in Rhode Island. Reported by: T Santibanez, PhD, L Barker, PhD, J Santoli, MD, C Bridges, MD, G Euler, DrPH, M McCauley, MTSC, National Immunization Program, CDC. Editorial Note:The findings in this report indicate that, during the first season in which ACIP encouraged childhood influenza vaccination, coverage was low and varied substantially among states. These first national estimates of childhood influenza-vaccination coverage provide a baseline for assessing implementation of the pediatric influenza-vaccination program recommended by ACIP. Coverage estimates for other routinely recommended childhood vaccines also vary across states and urban areas (6). Further study is needed to determine the reason for such variation and to identify useful strategies for increasing annual influenza vaccination among all groups of children (and adults) for whom the vaccine is now recommended (4). During the 2002--03 influenza season, influenza vaccination of healthy children aged 6--23 months was not yet covered by the Vaccines for Children Program (VFC) and also might not have been covered by the majority of private health plans. The ACIP encouragement rather than full recommendation and the lack of VFC coverage both likely contributed to the low coverage observed during the 2002--03 influenza season. However, beginning with the 2003--04 influenza season, ACIP voted to include in the VFC program annual influenza vaccination for all children aged 6--23 months and for household contacts of children aged <2 years. This expansion of VFC coverage for influenza vaccine enables providers to administer free influenza vaccine to the most vulnerable groups of children (i.e., Medicaid enrollees, uninsured children, American Indian/Alaska Native children, and certain children whose health insurance does not cover the cost of vaccination). More doses of influenza vaccine were produced and available than eventually were used for the 2002--03 influenza season, so problems of inadequate vaccine supply were unlikely to have contributed to the low vaccination coverage during that season. The vaccination rates described in this report might, in part, reflect implementation of the long-standing recommendation to administer influenza vaccine to children aged >6 months who have a high-risk condition or who live with a child or adult with a high-risk condition. However, this possibility could not be verified because NIS does not collect information on high-risk medical conditions or household contacts with high-risk conditions. In the United States, an estimated 5.5% of children aged 6--23 months have a high-risk condition for which influenza vaccination is recommended (7). Although national vaccination-coverage data are not available for groups at high risk, studies of specific populations have reported influenza coverage among children at high risk, ranging from 7% to 79% (4,8,9). In the 2004 NIS, information on risk status of children aged 19--35 months at the time of interview and of their household contacts is being collected for a subsample of households. Additional mechanisms are needed to assess coverage among children aged >35 months, including those who have one or more high-risk medical conditions or who live with a person at high risk (e.g., a child aged <2 years). Two decisions made during analysis might have influenced, in opposite directions, the vaccination-coverage estimates. First, analysis was limited to those vaccinations administered during September--December for the 1+ FLU measure and during September 1, 2002--January 31, 2003 (or date of interview if the interview occurred before January 31) for the fully vaccinated measure, although some vaccines might have been administered after these months and would not have been counted. This approach possibly reduced both measures of influenza-vaccination coverage reported here, particularly the estimate of fully vaccinated children, because difficulty in scheduling and returning for the second dose of influenza vaccine might delay receipt of the second dose until later in the influenza season. Second, measurement of vaccination coverage was restricted to children aged 6--23 months during the entire influenza-vaccination period of September--December. Children in this age group were eligible for vaccination under the ACIP encouragement for the entire period of assessment, so their caregivers and providers each had an equal amount of time to ensure vaccination. Therefore, this sample of children likely has higher vaccination coverage than children who were aged 6--23 months during only a portion of the 4-month vaccination interval, thereby potentially inflating the coverage estimate. The findings in this report are subject to at least three limitations. First, NIS is a telephone survey; although statistical adjustments compensate for expected differences in coverage in households without telephones, coverage bias might remain. Second, NIS relies on provider-verified vaccination histories; incomplete records and reporting might result in underestimates of coverage. Finally, because of sampling uncertainty and wide confidence intervals for many state and urban-area estimates from NIS, these estimates should be interpreted with caution. Influenza-vaccination coverage among children aged 6--23 months was low during the first year of the ACIP encouragement. For the 2004--05 influenza season, an ACIP recommendation replaces the encouragement that was in place previously (10); this change is expected to result in increased vaccination coverage. However, substantial work is needed to fully implement this new recommendation for children aged 6--23 months and household contacts of children aged <2 years and to reduce the number of preventable influenza-related hospitalizations among young children (2). References
Table 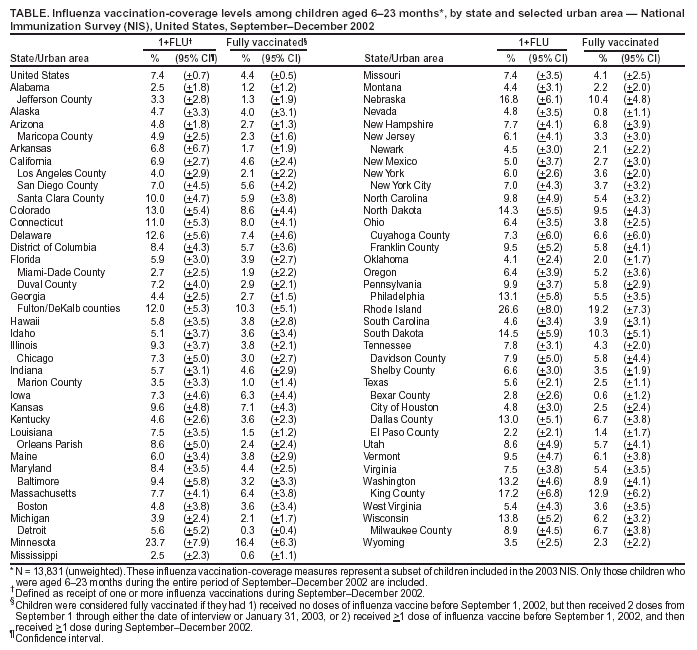 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/23/2004 |
|||||||||
This page last reviewed 9/23/2004
|