 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
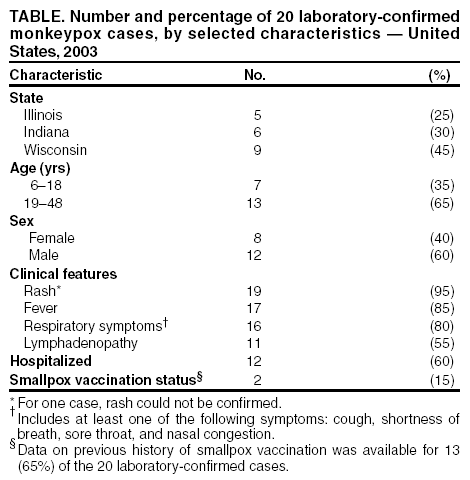
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Multistate Outbreak of Monkeypox --- Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003CDC and state and local health departments continue to investigate cases of monkeypox among persons who had close contact with wild or exotic mammalian pets or persons with monkeypox (1). This report updates epidemiologic, laboratory, and animal data for U.S. cases. Epidemiologic investigationAs of June 18, a total of 87 cases of monkeypox have been reported to CDC from Wisconsin (n = 38), Indiana (n = 24), Illinois (n = 19), Ohio (n = 4), Kansas (n = 1), and Missouri (n = 1). Of the 87 cases, 41 (47%) were among males. The median age for the 82 patients for whom age data were available was 28 years (range:1--55 years). Data on symptom onset were available for 78 persons (Figure). Among the 75 patients for whom data were available, 20 (27%) were hospitalized. The majority of patients were not seriously ill; some were hospitalized to facilitate proper isolation. Of the 87 monkeypox cases, 20 (23%) were laboratory confirmed at CDC (Table). Among these 20 patients, one was a child hospitalized with severe encephalitis 3 days after developing a vesicular rash, which was originally thought to be varicella-zoster virus (VZV). However, diagnostic testing for VZV and for herpes simplex virus in serum, cerebrospinal fluid, and skin lesion biopsy was negative. A skin lesion biopsy was positive for monkeypox DNA by polymerase chain reaction (PCR) and for orthopox antigens by immunohistochemical (IHC) testing. The majority of patients had direct or close contact with wild or exotic mammals such as prairie dogs (Cynomys sp.). In one instance, 28 children attending a day care facility in Indiana were potentially exposed to two prairie dogs that subsequently became ill and died; 12 (43%) reported handling or petting the prairie dogs, and seven (25%) subsequently became ill with symptoms consistent with monkeypox infection. Laboratory evaluation of these children is in progress. Laboratory InvestigationClinical specimens obtained from 82 patients in Illinois, Indiana, Ohio, and Wisconsin were forwarded to CDC for testing. Twenty (74%) of 27 patients with skin rash-lesion specimens were laboratory confirmed for monkeypox by viral isolation, PCR, electron microscopy, and/or IHC; four were negative for monkeypox virus; one patient was found to have varicella by PCR testing; and two are pending. Two health-care workers in Wisconsin who were suspected initially of acquiring disease by human-to-human transmission had no evidence of monkeypox-specific DNA signatures in blood and nasopharyngeal and/or oropharyngeal swabs; culture results are pending. These persons did not have a rash, and IgM testing has not revealed any anti-orthopoxvirus immune reactivity. Animal InvestigationTraceback investigations of animals are ongoing to identify how monkeypox virus was introducted into the United States. Preliminary results have determined that an animal vendor in Wisconsin (distributor A) sold prairie dogs to the index patient in Wisconsin; this vendor had obtained prairie dogs from an animal vendor in Illinois (distributor B), who had housed prairie dogs and Gambian giant rats (Cricetomys sp.) in close proximity. Because Gambian giant rats often are imported from regions of Africa where monkeypox is endemic, traceback investigations of the Gambian giant rats were initiated. These investigations identified a shipment of animals from Ghana, including Gambian giant rats that were delivered to a Texas animal importer (distributor C) on April 9. Distributor C's Gambian giant rats were sold subsequently to an Iowa animal vendor on April 15 (distributor D) who in turn supplied them to distributor B. The shipment of animals from Ghana contained approximately 800 small mammals of nine different species, including six genera of African rodents that might have been the source of introduction of monkeypox. These rodent genera included rope squirrels (Funiscuirus sp.), tree squirrels (Heliosciurus sp.), Gambian giant rats, brushtail porcupines (Atherurus sp.), dormice (Graphiurus sp.), and striped mice (Hybomys sp.). Laboratory testing of animals from the April 9 importation from Africa is underway to determine which, if any, animals in the shipment might have introduced the virus into the United States. On the basis of the epidemiologic link between the shipment from Ghana and distributor B, trace-forward investigations have been initiated to locate animal vendors and owners who purchased imported African rodents from the April 9 shipment or purchased prairie dogs from distributors A, B, C, and D after April 15. In addition to routine sales by animal vendors, animals also were sold or traded at "swap meets" (i.e., gatherings of animal traders, exhibitors, and buyers). An investigation of distributor B revealed that infected prairie dogs from this animal vendor might have been sold or traded at swap meets to unidentified buyers in Schaumburg, Illinois, on April 20, May 3, and May 18; Indianapolis, Indiana, on April 27 and May 18; and Columbus, Ohio, on April 19. In addition, distributor A sold infected prairie dogs at a swap meet in Wausau, Wisconsin, on May 11. In several instances, identifying individuals who purchased animals has been impossible. Invoices and other records are incomplete for many of these sales, especially those transacted at swap meets. Reported by: CW Langkop, MSPH, C Austin, PhD, M Dworkin, MD, K Kelly, Illinois Dept of Public Health. H Messersmith, MPH, R Teclaw, PhD, J Howell, MPH, J Michael, MS, P Pontones, MA, Indiana State Dept of Health. Monkeypox Investigation Team, G Pezzino, MD, GR Hansen, MPH, Kansas Dept of Health and Environment. Monkeypox Investigation Team, MV Wegner, MD, JJ Kazmierczak, DVM, C Williams, DVM, DR Croft, MD, S Ahrabi-Fard, MS, L Will, PhD, HH Bostrom, JP Davis, MD, Wisconsin Dept of Health and Family Svcs., Monkeypox Investigation Team; A Fleischauer, PhD, M Sotir, PhD, G Huhn, MD, R Kanwal, MD, J Kile, DVM, J Sejvar, MD, EIS officers, CDC. Editorial Note:Preliminary findings from these investigations suggest that the primary route of monkeypox transmission to humans is from close contact with infected wild and exotic mammalian pets. Person-to-person transmission has not been identified in this outbreak. Investigations are underway to assess the possibility of secondary transmission among health-care workers and household contacts exposed to patients with laboratory-confirmed monkeypox infection. Compared with previous reports of monkeypox among persons in central Africa (2), the illness associated with the current outbreak in the United States has been relatively mild. Monkeypox infection in adults has been described rarely in Africa; among adults, previous vaccination against smallpox might attenuate clinical illness (3). The report of encephalitis in a child indicates the potentially serious consequences of the disease. Because suspected cases of monkeypox might actually represent varicella infections, patients should be assessed for history of varicella or having received varicella vaccine. Rash illness suspected to be monkeypox should be confirmed by laboratory evaluation, particularly if use of smallpox vaccine is being considered for purposes of monkeypox outbreak control. CDC has issued interim recommendations for use of smallpox vaccine, cidofovir, and vaccinia immune globulin (VIG) for prevention and treatment in the setting of outbreaks of monkeypox infections (4). Health-care providers, veterinarians, and public health officials who suspect monkeypox in animals or humans should report such cases to their state and local health departments. CDC requests that reports of suspect cases from state health departments be directed to the CDC Emergency Operations Center, telephone 770-488-7100. Additional information about monkeypox, including a revised interim case definition (Box), is available at http://www.cdc.gov/ncidod/monkeypox. References
 Return to top. Figure 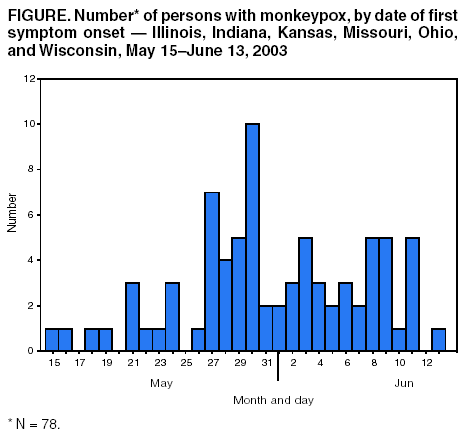 Return to top. Box 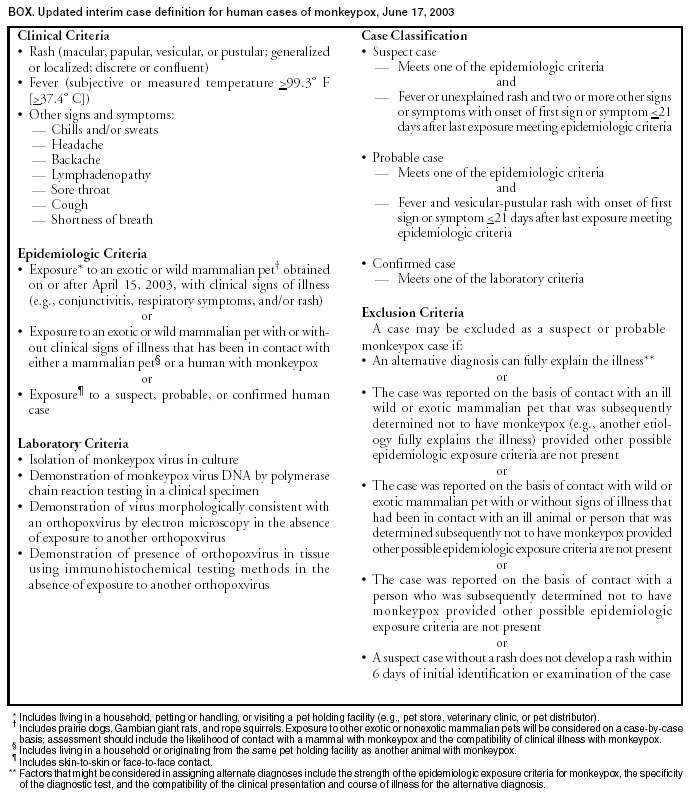 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 6/19/2003 |
|||||||||
This page last reviewed 6/19/2003
|