 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Hepatitis A Vaccination Programs in Communities with High Rates of Hepatitis AIn June 1995, the Public Health Service Advisory Committee on Immunization Practices (ACIP) issued recommendations about the use of hepatitis A vaccine for the prevention and control of hepatitis A (1). In communities with high rates of hepatitis A and periodic outbreaks, the ACIP recommends routine vaccination of young children and catch-up vaccination of previously unvaccinated older children (1). This report describes hepatitis A vaccination programs initiated to control ongoing outbreaks and prevent future outbreaks in two communities with high rates of hepatitis A. Preliminary epidemiologic data indicate that the program in one area may have decreased the magnitude and duration of a predicted outbreak. The incidence of hepatitis A in other areas will require long-term monitoring to determine the effect of the vaccination program. Northern Plains Indians Outbreaks of hepatitis A occur periodically (i.e., at 5-7 year intervals) in many American Indian and Alaskan Native communities and typically last for 2-3 years. Cases primarily occur among children aged less than 15 years, 30%-40% of children become infected before age 5 years, and approximately 80% are immune after age 12 years (2). During 1995-1996, the Indian Health Service (IHS), in collaboration with state health departments and tribal health authorities, implemented hepatitis A vaccination programs on several Northern Plains Indian reservations. On reservations with ongoing outbreaks, catch-up vaccination of children aged 5-12 years was conducted through vaccination clinics held in schools, and preschool- and school-aged children were vaccinated in IHS clinics. In some areas, preschool-aged children also received vaccine through the Head Start program. On reservations without ongoing outbreaks, hepatitis A vaccine was available to children aged 2-12 years who visited IHS clinics. To promote the program, news media releases and public service announcements were issued, and information was sent home with schoolchildren. In addition, vaccination program staff met with and received input and support from tribal groups, community service leaders, and school staff. To estimate vaccination coverage among children in the target population, IHS and CDC reviewed medical records of a random sample of 670 (6%) of the estimated 10,600 children aged 2-12 years in three IHS service units (service units 1 and 2 correspond to reservations 1 and 2, and service unit 3 is an urban area) approximately 1 year after implementation of the vaccination programs; the Clinic Assessment Software Application was used in the review (3). Records without hepatitis A vaccination information were cross-checked for vaccination status using other vaccination databases and school records. Estimated first dose vaccination coverage was 71% (95% confidence interval {CI}=69%-74%) in unit 1, 27% (95% CI=24%-30%) in unit 2, and 18% (95% CI=14%-23%) in unit 3. Of all unvaccinated children, 77% (95% CI=74%-80%) had visited a clinic during the preceding year for a condition for which vaccination was not contraindicated. To evaluate the characteristics of parents/guardians associated with participation in the vaccination program, interviews of a sample of 160 parents/guardians of children aged 2-12 years on reservation 1 were conducted. In each area of the reservation, interviewers and tribal health staff responsible for that area drove through the area and visited households identified by staff as including a child in the targeted age group. Of the 160 survey participants, 121 (76%) had had their children vaccinated. Of these children, 63 (52%) had been vaccinated at school, 54 (45%) at a clinic, and four (3%) at other sites. Most (144 {90%}) survey participants had received information about the vaccination program, primarily from schools, public health nurses, clinics, and radio broadcasts. Of the 39 participants whose children were not vaccinated, 27 (69%) reported they did want their child to be vaccinated. The most frequently reported reason (80%) for the child not being vaccinated at school was that the parent/guardian wanted to be present at the time of vaccination. Based on previous patterns of hepatitis A outbreaks on reservations 1 and 2, outbreaks were predicted for these areas in 1995-1996. During 1970-1994, a total of 95-320 cases were reported during previous outbreaks on reservation 1; in comparison, during 1995-1996, a total of 20 cases of hepatitis A were reported on reservation 1 (Figure_1). These cases occurred before or early in the course of the vaccination program; no cases have been reported since June 1996. On reservation 2, a total of 42 cases were reported during 1995-1996, compared with 54-116 cases during previous outbreaks. Most cases reported during 1995-1996 occurred before the vaccination program was started. Tradition-Observant Jewish Community, Brooklyn, New York Hepatitis A historically has been endemic among tradition-observant Jews in Brooklyn, New York (estimated number of persons: 90,000). During 1991-1995, two large outbreaks occurred in this community; in 1991, the reported rate was 157 cases per 100,000 population, and during 1995, the rate was 243. During both outbreaks, the rates were highest among children aged less than 10 years. To help prevent and control these outbreaks, in mid-1995 the New York City Department of Health (NYCDOH), in collaboration with local physicians and the community, initiated a hepatitis A vaccination program especially targeting an estimated 3700 children aged 2-5 years who resided in or attended private, religious schools in the community. Of 21 pediatric practices serving this community, 18 practices participated in the program and received free hepatitis A vaccine, initially from NYCDOH and later through the Vaccines For Children (VFC) program. The vaccination program was promoted through letters and fact sheets distributed to parents by schools, announcements on local radio stations and in newspapers, and in meetings with local pediatricians. From September 1995 through August 1996, a total of 12,530 doses of hepatitis A vaccine, including 7530 doses obtained through the VFC program, was distributed to the community. Of the 14 cases reported in 1996, two occurred among children in the age group targeted for vaccination; neither had received hepatitis A vaccine. To assess the impact of the campaign on physician practices, the NYCDOH distributed a survey to all 18 of the participating practices in May 1996; a total of 16 practices completed the survey. Of the 16, eight reported that at least 50% of their patient population was aged less than 5 years. Since the beginning of the campaign, all the pediatric practices surveyed reported that they routinely administered hepatitis A vaccine to the children in the targeted age group; 38% reported that they also administered vaccine to persons aged 5-19 years in their practice. Reported by: T Welty, MD, K Darling, M Magera, Aberdeen Area Office, Indian Health Svc; J Cheek, MD, Headquarters West, Indian Health Svc. L Volmer, L Schaefer, S Gregg, S Lance, DVM, State Epidemiologist, South Dakota State Dept of Health. S Schulman, MD, A Hakim, MD, R Adler, MD, G Bard, MD, D Diamond, MD, K Feuerstein, MD, C Gelbfish, MD, B Krieger, MD, E Mandel, MD, L Mogilner, MD, A Nussbaum, MD, T Powers, MD, N Ruttner, MD, O Roth, MD, S Styler, MD, Maimonides Medical Center; M Lew, MD, Methodist Hospital; V Santilli, MD, Brook-Island Pediatric Group, New York City; J Kellachan, MPH, M Layton, MD, C Whitman, MD, S Friedman, MD, B Mojica, MD, New York City Dept of Health. Hepatitis Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: Communities with high rates of hepatitis A are characterized by epidemics that occur with regular periodicity and by a high incidence of cases among children aged less than or equal to 15 years (1). This report described two examples of hepatitis A vaccination programs that are being implemented in some communities with high rates of hepatitis A. The effectiveness of these programs in reaching the targeted population has varied. Among the communities of Northern Plains Indians, a high level of vaccination coverage was achieved on reservation 1 by providing vaccine through IHS clinics and schools. Only small proportions of the target populations were vaccinated on reservation 2, despite an ongoing outbreak, and in the urban area receiving services from unit 3, where few cases were reported and vaccine was available only in the clinic. The high proportion of unvaccinated children surveyed who had visited a facility during the preceding year indicates that missed opportunities for vaccination were common. The vaccination program in Brooklyn demonstrates that community physicians will provide hepatitis A vaccine to patients in their practices. Although vaccination coverage cannot be accurately estimated, the number of doses distributed suggests that a substantial proportion of the target population was vaccinated at these physicians' offices. Widespread vaccination in communities with high rates of hepatitis A can prevent future outbreaks and control ongoing outbreaks (4). The vaccination program on reservation 1 was initiated shortly after cases had started to occur and may have prevented a larger outbreak. Because the outbreaks in Brooklyn and on reservation 2 had been ongoing for at least 1 year when the vaccination programs were initiated, their effect on the ongoing outbreaks could not be readily assessed. Hepatitis A vaccination programs represent an important strategy for preventing morbidity and mortality associated with cyclic hepatitis A epidemics in communities with high rates of disease. Programs should be implemented in these communities through clinics, physicians' offices, and other sites where vaccinations are administered, and in communities with ongoing outbreaks, school-based vaccination programs should be considered. Vaccine can be ordered through the VFC program for all VFC-eligible children aged 2-18 years. Because hepatitis A vaccine is licensed for children aged greater than or equal to 2 years, innovative strategies must be developed to reach preschool- and school-aged children. In communities without ongoing outbreaks, community members and health-care providers should be educated about the epidemiology of hepatitis A in their communities and the rationale for hepatitis A vaccination. Vaccination of successive cohorts of 2-year-old children and catch-up vaccination of older children will help prevent future outbreaks in these communities. References
Figure_1 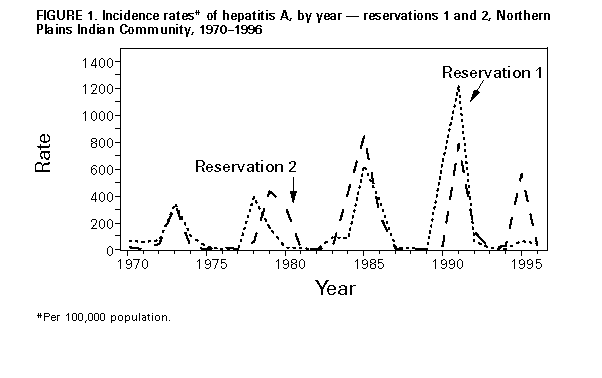 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|